Combustion of amines. Amines
Based on the nature of hydrocarbon substituents, amines are divided into
General structural features of amines
Just like in the ammonia molecule, in the molecule of any amine the nitrogen atom has a lone electron pair directed to one of the vertices of the distorted tetrahedron:
For this reason, amines, like ammonia, have significantly expressed basic properties.
Thus, amines, similar to ammonia, react reversibly with water, forming weak bases:
The bond between the hydrogen cation and the nitrogen atom in the amine molecule is realized using a donor-acceptor mechanism due to the lone electron pair of the nitrogen atom. Saturated amines are stronger bases compared to ammonia, because in such amines, hydrocarbon substituents have a positive inductive (+I) effect. In this regard, the electron density on the nitrogen atom increases, which facilitates its interaction with the H + cation.
Aromatic amines, if the amino group is directly connected to the aromatic ring, exhibit weaker basic properties compared to ammonia. This is due to the fact that the lone electron pair of the nitrogen atom is shifted towards the aromatic π-system of the benzene ring, as a result of which the electron density on the nitrogen atom decreases. In turn, this leads to a decrease in basic properties, in particular the ability to interact with water. For example, aniline reacts only with strong acids, but practically does not react with water.
Chemical properties of saturated amines
As already mentioned, amines react reversibly with water:
Aqueous solutions of amines have an alkaline reaction due to the dissociation of the resulting bases:
Saturated amines react with water better than ammonia due to their stronger basic properties.
The basic properties of saturated amines increase in the series.
Secondary saturated amines are stronger bases than primary saturated amines, which in turn are stronger bases than ammonia. As for the basic properties of tertiary amines, if we are talking about reactions in aqueous solutions, then the basic properties of tertiary amines are expressed much worse than those of secondary amines, and even slightly worse than those of primary ones. This is due to steric hindrances, which significantly affect the rate of amine protonation. In other words, three substituents “block” the nitrogen atom and interfere with its interaction with H + cations.
Interaction with acids
Both free saturated amines and their aqueous solutions react with acids. In this case, salts are formed:
Since the basic properties of saturated amines are more pronounced than those of ammonia, such amines react even with weak acids, such as carbonic acid:
Amine salts are solids, highly soluble in water and poorly soluble in non-polar organic solvents. The interaction of amine salts with alkalis leads to the release of free amines, similar to the displacement of ammonia when alkalis act on ammonium salts:
2. Primary saturated amines react with nitrous acid to form the corresponding alcohols, nitrogen N2 and water. For example:
A characteristic feature This reaction is the formation of nitrogen gas, and therefore it is qualitative for primary amines and is used to distinguish them from secondary and tertiary amines. It should be noted that most often this reaction is carried out by mixing the amine not with a solution of nitrous acid itself, but with a solution of a salt of nitrous acid (nitrite) and then adding a strong mineral acid to this mixture. When nitrites interact with strong mineral acids, nitrous acid is formed, which then reacts with the amine:
Secondary amines give similar conditions oily liquids, so-called N-nitrosamines, but this reaction in real tasks There is no Unified State Examination in chemistry. Tertiary amines do not react with nitrous acid.
Complete combustion of any amines leads to the formation of carbon dioxide, water and nitrogen:
Interaction with haloalkanes
It is noteworthy that exactly the same salt is obtained by the action of hydrogen chloride on a more substituted amine. In our case, when hydrogen chloride reacts with dimethylamine:
Preparation of amines:
1) Alkylation of ammonia with haloalkanes:
In case of ammonia deficiency, its salt is obtained instead of amine:
2) Reduction by metals (to hydrogen in the activity series) in an acidic environment:
followed by treatment of the solution with alkali to release the free amine:
3) The reaction of ammonia with alcohols when passing their mixture through heated aluminum oxide. Depending on the alcohol/amine proportions, primary, secondary or tertiary amines are formed:
Chemical properties of aniline
Aniline – trivial name aminobenzene having the formula:
As can be seen from the illustration, in the aniline molecule the amino group is directly connected to the aromatic ring. Such amines, as already mentioned, have much less pronounced basic properties than ammonia. Thus, in particular, aniline practically does not react with water and weak acids such as carbonic acid.
Reaction of aniline with acids
Aniline reacts with strong and medium strength inorganic acids. In this case, phenylammonium salts are formed:
Reaction of aniline with halogens
As was already said at the very beginning of this chapter, the amino group in aromatic amines is drawn into the aromatic ring, which in turn reduces the electron density on the nitrogen atom, and as a result increases it in the aromatic ring. An increase in electron density in the aromatic ring leads to the fact that electrophilic substitution reactions, in particular reactions with halogens, proceed much more easily, especially in the ortho and para positions relative to the amino group. Thus, aniline easily reacts with bromine water, forming a white precipitate of 2,4,6-tribromoaniline:
This reaction is qualitative for aniline and often makes it possible to identify it among others organic compounds.
Reaction of aniline with nitrous acid
Aniline reacts with nitrous acid, but due to the specificity and complexity of this reaction, it does not appear in the real Unified State Exam in chemistry.
Aniline alkylation reactions
Using sequential alkylation of aniline at the nitrogen atom with halogenated hydrocarbons, secondary and tertiary amines can be obtained:
Obtaining aniline
1. Reduction of nitrobenzene by metals in the presence of strong non-oxidizing acids:
C 6 H 5 -NO 2 + 3Fe + 7HCl = +Cl- + 3FeCl 2 + 2H 2 O
Cl - + NaOH = C 6 H 5 -NH 2 + NaCl + H 2 O
Any metals located before hydrogen in the activity series can be used as metals.
Reaction of chlorobenzene with ammonia:
C 6 H 5 −Cl + 2NH 3 → C 6 H 5 NH 2 + NH 4 Cl
Chemical properties of amino acids
Amino acids are compounds whose molecules contain two types of functional groups - amino (-NH 2) and carboxy- (-COOH) groups.
In other words, amino acids can be considered as derivatives of carboxylic acids, in the molecules of which one or more hydrogen atoms are replaced by amino groups.
Thus, general formula amino acids can be written as (NH 2) x R(COOH) y, where x and y are most often equal to one or two.
Since amino acid molecules contain both an amino group and a carboxyl group, they exhibit Chemical properties similar to both amines and carboxylic acids.
Acidic properties of amino acids
Formation of salts with alkalis and alkali metal carbonates
Esterification of amino acids
Amino acids can react with esterification with alcohols:
NH 2 CH 2 COOH + CH 3 OH → NH 2 CH 2 COOCH 3 + H 2 O
Basic properties of amino acids
1. Formation of salts when interacting with acids
NH 2 CH 2 COOH + HCl → + Cl —
2. Interaction with nitrous acid
NH 2 -CH 2 -COOH + HNO 2 → HO-CH 2 -COOH + N 2 + H 2 O
Note: interaction with nitrous acid proceeds in the same way as with primary amines
3. Alkylation
NH 2 CH 2 COOH + CH 3 I → + I —
4. Interaction of amino acids with each other
Amino acids can react with each other to form peptides - compounds containing in their molecules the peptide bond –C(O)-NH-
At the same time, it should be noted that in the case of a reaction between two different amino acids, without observing some specific synthesis conditions, the formation of different dipeptides occurs simultaneously. So, for example, instead of the reaction of glycine with alanine above, leading to glycylananine, the reaction leading to alanylglycine can occur:
In addition, the glycine molecule does not necessarily react with the alanine molecule. Peptization reactions also occur between glycine molecules:
And alanine:
In addition, since the molecules of the resulting peptides, like the original amino acid molecules, contain amino groups and carboxyl groups, the peptides themselves can react with amino acids and other peptides due to the formation of new peptide bonds.
Individual amino acids are used to produce synthetic polypeptides or so-called polyamide fibers. Thus, in particular, using the polycondensation of 6-aminohexane (ε-aminocaproic) acid, nylon is synthesized in industry:
The resulting nylon resin is used to produce textile fibers and plastics.
Formation of internal salts of amino acids in aqueous solution
In aqueous solutions, amino acids exist predominantly in the form of internal salts - bipolar ions (zwitterions):
Obtaining amino acids
1) Reaction of chlorinated carboxylic acids with ammonia:
Cl-CH 2 -COOH + 2NH 3 = NH 2 -CH 2 -COOH + NH 4 Cl
2) Breakdown (hydrolysis) of proteins under the action of solutions of strong mineral acids and alkalis.
Amines- organic derivatives of ammonia, in the molecule of which one, two or all three hydrogen atoms are replaced by a carbon residue.
There are usually three types of amines:
Amines in which the amino group is bonded directly to an aromatic ring are called aromatic amines.
The simplest representative of these compounds is aminobenzene, or aniline:

Basic distinctive feature electronic structure amines is the presence of a lone electron pair on the nitrogen atom included in the functional group. This causes amines to exhibit the properties of bases.
There are ions that are the product of the formal replacement of all hydrogen atoms in the ammonium ion by a hydrocarbon radical:

These ions are found in salts similar to ammonium salts. They are called quaternary ammonium salts.
Isomerism and nomenclature of amines
1. Amines are characterized by structural isomerism:
A) carbon skeleton isomerism:

b) isomerism of the position of the functional group:
2. Primary, secondary and tertiary amines are isomeric to each other (interclass isomerism):

As can be seen from the examples given, in order to name an amine, the substituents associated with the nitrogen atom are listed (in order of precedence) and the suffix is added - amine.
Physical properties of amines
The simplest amines (methylamine, dimethylamine, trimethylamine) - gaseous substances. The remaining lower amines are liquids that dissolve well in water. They have a characteristic odor reminiscent of ammonia.
Primary and secondary amines are capable of forming hydrogen bonds. This leads to a noticeable increase in their boiling points compared to compounds that have the same molecular weight but are unable to form hydrogen bonds.
Aniline is an oily liquid, sparingly soluble in water, boiling at a temperature of 184 °C.
Chemical properties of amines
The chemical properties of amines are determined mainly by the presence of a lone electron pair on the nitrogen atom.
Amines as bases. The nitrogen atom of the amino group, like the nitrogen atom in the ammonia molecule, due to the lone pair of electrons can form covalent bond according to the donor-acceptor mechanism, acting as a donor. In this regard, amines, like ammonia, are capable of attaching a hydrogen cation, i.e., acting as a base:

1. Reaction of amions with water leads to the formation of hydroxide ions:
2. Reaction with acids. Ammonia reacts with acids to form ammonium salts. Amines are also capable of reacting with acids:

The basic properties of aliphatic amines are more pronounced than those of ammonia. This is due to the presence of one or more donor alkyl substituents, the positive inductive effect of which increases the electron density on the nitrogen atom. An increase in electron density turns nitrogen into a stronger electron pair donor, which improves its basic properties:

Amion combustion. Amines burn in air to form carbon dioxide, water and nitrogen:
Application of amines
Amines are widely used to produce drugs and polymer materials. Aniline is the most important compound of this class, which is used for the production of aniline dyes, drugs (sulfonamide drugs), and polymeric materials (aniline formaldehyde resins).



AMINES- a class of compounds that are organic derivatives of ammonia in which one, two or three hydrogen atoms are replaced by organic groups. A distinctive feature is the presence of the R–N fragment<, где R – органическая группа.
The classification of amines is varied and is determined by which structural feature is taken as a basis.
Depending on the number of organic groups associated with the nitrogen atom, there are:
primary amines - one organic group at nitrogen RNH2
secondary amines - two organic groups on nitrogen R2NH, organic groups can be different R"R"NH
tertiary amines - three organic groups on nitrogen R3N or R"R"R""N
Based on the type of organic group associated with nitrogen, aliphatic СH3 – N are distinguished< и ароматические С 6 H5 – N< амины, возможны и смешанные варианты.
Based on the number of amino groups in the molecule, amines are divided into monoamines CH3 - NH2, diamines H2N(CH2) 2 NH2, triamines, etc.
Chemical properties of amines. The distinctive ability of amines is to attach neutral molecules (for example, hydrogen halides HHal, with the formation of organoammonium salts, similar to ammonium salts in inorganic chemistry. To form a new bond, nitrogen provides a lone electron pair, acting as a donor. The H + proton involved in the formation of the bond (from the hydrogen halide) plays the role of an acceptor (receiver), such a bond is called donor-acceptor (Fig. 1).The resulting covalent N–H bond is completely equivalent to the bonds present in the amine
Tertiary amines also add HCl, but when the resulting salt is heated in an acid solution, it decomposes, and R is cleaved from the N atom:
(C 2 H 5) 3 N+ HCl [(C 2 H 5) 3 N H]Cl
[(C 2 H 5) 3 N H]Cl (C 2 H 5) 2 N H + C 2 H 5 Cl
When comparing these two reactions, it is clear that the C2H5 group and H seem to change places, as a result, a secondary amine is formed from a tertiary amine.
Dissolving in water, amines capture a proton in the same way, as a result OH – ions appear in the solution, which corresponds to the formation of an alkaline environment, which can be detected using conventional indicators.
C2H5 N H2 + H2O + + OH–
With the formation of a donor-acceptor bond, amines can add not only HCl, but also haloalkyl RCl, thereby forming a new N–R bond, which is also equivalent to the existing ones. If we take a tertiary amine as the starting material, we obtain a tetraalkylammonium salt (four R groups on one N atom):
(C 2 H 5) 3 N+ C 2 H 5 I [(C 2 H 5) 4 N]I
These salts, dissolving in water and some organic solvents, dissociate (disintegrate), forming ions:
[(C2H5) 4 N]I [(C2H5) 4 N] + + I–
Such solutions, like all solutions containing ions, conduct electric current. In tetraalkylammonium salts, the halogen can be replaced with an HO group:
[(CH 3) 4 N]Cl + AgOH [(CH 3) 4 N]OH + AgCl
The resulting tetramethylammonium hydroxide is a strong base with properties similar to alkalis.
Primary and secondary amines react with nitrous acid HON=O, but they react in different ways. Primary alcohols are formed from primary amines:
C2H5 N H2+H N O2 C2H5OH + N 2 +H2O
Unlike primary amines, secondary amines form yellow, poorly soluble nitrosamines with nitrous acid - compounds containing the fragment >N–N = O:
(C 2 H 5) 2 N H+H N O 2 (C 2 H 5) 2 N–N=O + H2O
Tertiary amines do not react with nitrous acid at ordinary temperatures, so nitrous acid is a reagent that allows one to distinguish between primary, secondary and tertiary amines.
When amines condense with carboxylic acids acid amides are formed - compounds with the –C(O)N fragment< (рис. 2А). Если в качестве исходных соединений взять диамин и дикарбоновую кислоту (соединения, содержащие соответственно две амино- и две карбоксильные группы, соответственно), то они взаимодействуют по такой же схеме, но поскольку каждое соединение содержит две реагирующие группы, то образуется полимерная цепь, содержащая амидные группы (рис. 2Б). Такие полимеры называют полиамидами.
The condensation of amines with aldehydes and ketones leads to the formation of so-called Schiff bases - compounds containing the –N=C fragment< (рис. 2В). На схеме В видно, что для образования двойной связи между N и С азот должен предоставить два атома Н (для образования конденсационной воды), следовательно, в такой реакции могут участвовать только первичные амины RNH2.
When primary amines interact with phosgene Cl2C=O, compounds with the –N=C=O group are formed, called isocyanates (Fig. 2D, preparation of a compound with two isocyanate groups).

Among the aromatic amines, the most famous is aniline (phenylamine) C 6 H 5 NH 2. Its properties are similar to aliphatic amines, but its basicity is less pronounced - it does not form an alkaline environment in aqueous solutions. Like aliphatic amines, with strong mineral acids it can form ammonium salts [C 6 H 5 NH 3 ] + Cl–. When aniline reacts with nitrous acid (in the presence of HCl), a diazo compound containing the R–N=N fragment is formed; it is obtained in the form of an ionic salt called the diazonium salt (Fig. 3A). Thus, the interaction with nitrous acid does not proceed in the same way as in the case of aliphatic amines. The benzene ring in aniline has a reactivity characteristic of aromatic compounds (see AROMATICITY); during halogenation, hydrogen atoms in the ortho and para positions to the amino group are replaced, resulting in chloroanilines with varying degrees of substitution (Fig. 3B). The action of sulfuric acid leads to sulfonation in the para position to the amino group, the so-called sulfanilic acid is formed (Fig. 3B).

Since amines, being derivatives of ammonia, have a structure similar to it (i.e. they have a lone pair of electrons in the nitrogen atom), they exhibit properties similar to it. Those. amines, like ammonia, are bases because the nitrogen atom can provide an electron pair to form bonds with electron-deficient species through a donor-acceptor mechanism (meeting the Lewis definition of basicity).
I. Properties of amines as bases (proton acceptors)
1. Aqueous solutions of aliphatic amines exhibit an alkaline reaction, because when they interact with water, alkyl ammonium hydroxides are formed, similar to ammonium hydroxide:
CH 3 NH 2 + H 2 O CH 3 NH 3 + + OH −


Aniline practically does not react with water.
Aqueous solutions are alkaline:
The proton bond with an amine, as with ammonia, is formed by a donor-acceptor mechanism due to the lone electron pair of the nitrogen atom.
Aliphatic amines are stronger bases than ammonia because alkyl radicals increase the electron density on the nitrogen atom due to + I-effect. For this reason, the electron pair of the nitrogen atom is held less tightly and interacts more easily with the proton.
2. Interacting with acids, amines form salts:
C 6 H 5 NH 2 + HCl → (C 6 H 5 NH 3) Cl
phenylammonium chloride

2CH 3 NH 2 + H 2 SO 4 → (CH 3 NH 3) 2 SO 4
methyl ammonium sulfate
Amine salts are solids that are highly soluble in water and poorly soluble in non-polar liquids. When reacting with alkalis, free amines are released:
Aromatic amines are weaker bases than ammonia because the lone electron pair of the nitrogen atom is shifted towards the benzene ring, conjugating with the π electrons of the aromatic ring, which reduces the electron density on the nitrogen atom (-M effect). On the contrary, the alkyl group is a good donor of electron density (+I-effect).
 or
or 

A decrease in electron density on the nitrogen atom leads to a decrease in the ability to abstract protons from weak acids. Therefore, aniline interacts only with strong acids (HCl, H 2 SO 4), and its aqueous solution does not stain litmus in Blue colour.
The nitrogen atom in amine molecules has a lone pair of electrons, which can participate in the formation of bonds according to the donor-acceptor mechanism.
aniline ammonia primary amine secondary amine tertiary amine
the electron density on the nitrogen atom increases.
Due to the presence of a lone pair of electrons in the molecules, amines, like ammonia, exhibit basic properties.
aniline ammonia primary amine secondary amine
the basic properties are enhanced due to the influence of the type and number of radicals.
C6H5NH2< NH 3 < RNH 2 < R 2 NH < R 3 N (в газовой фазе)
II. Amine oxidation
Amines, especially aromatic ones, are easily oxidized in air. Unlike ammonia, they can ignite from an open flame. Aromatic amines spontaneously oxidize in air. Thus, aniline quickly turns brown in air due to oxidation.
4СH 3 NH 2 + 9O 2 → 4CO 2 + 10H 2 O + 2N 2
4C 6 H 5 NH 2 + 31O 2 → 24CO 2 + 14H 2 O + 2N 2
III. Interaction with nitrous acid
Nitrous acid HNO 2 is an unstable compound. Therefore, it is used only at the time of selection. HNO 2 is formed, like all weak acids, by the action of its salt (nitrite) strong acid:
KNO 2 + HCl → HNO 2 + KCl
or NO 2 − + H + → HNO 2
The structure of the reaction products with nitrous acid depends on the nature of the amine. Therefore, this reaction is used to distinguish between primary, secondary and tertiary amines.
· Primary aliphatic amines form alcohols with HNO 2:
R-NH 2 + HNO 2 → R-OH + N 2 + H 2 O
- Of great importance is the reaction of diazotization of primary aromatic amines under the action of nitrous acid, obtained by the reaction of sodium nitrite with hydrochloric acid. And subsequently phenol is formed:


· Secondary amines (aliphatic and aromatic) under the influence of HNO 2 are converted into N-nitroso derivatives (substances with a characteristic odor):
R 2 NH + H-O-N=O → R 2 N-N=O + H 2 O
alkylnitrosamine
· Reaction with tertiary amines leads to the formation of unstable salts and is of no practical importance.
IV. Special properties:
1. Formation of complex compounds with transition metals:
2. Addition of alkyl halides Amines add haloalkanes to form a salt:

By treating the resulting salt with alkali, you can obtain a free amine:

V. Aromatic electrophilic substitution in aromatic amines (reaction of aniline with bromine water or nitric acid):
In aromatic amines, the amino group facilitates substitution at the ortho and para positions of the benzene ring. Therefore, aniline halogenation occurs quickly and in the absence of catalysts, and three hydrogen atoms of the benzene ring are replaced at once, and a white precipitate of 2,4,6-tribromoaniline precipitates:

This reaction with bromine water is used as a qualitative reaction for aniline.
These reactions (bromination and nitration) predominantly produce ortho- And pair- derivatives.
4. Methods for producing amines.
1. Hoffmann reaction. One of the first methods for producing primary amines was the alkylation of ammonia with alkyl halides:
This is not the best method, since the result is a mixture of amines of all degrees of substitution:
etc. Not only alkyl halides, but also alcohols can act as alkylating agents. To do this, a mixture of ammonia and alcohol is passed over aluminum oxide at high temperature.
2. Zinin's reaction- a convenient way to obtain aromatic amines by reducing aromatic nitro compounds. The following are used as reducing agents: H 2 (on a catalyst). Sometimes hydrogen is generated directly at the time of the reaction, for which metals (zinc, iron) are treated with dilute acid.

2HCl + Fe (chips) → FeCl 2 + 2H
C 6 H 5 NO 2 + 6[H] C 6 H 5 NH 2 + 2H 2 O.
In industry, this reaction occurs when nitrobenzene is heated with steam in the presence of iron. In the laboratory, hydrogen “at the moment of release” is formed by the reaction of zinc with alkali or iron with hydrochloric acid. In the latter case, anilinium chloride is formed.
3. Reduction of nitriles. Use LiAlH 4:
4. Enzymatic decarboxylation of amino acids:

5. Application of amines.
Amines are used in the pharmaceutical industry and organic synthesis (CH 3 NH 2, (CH 3) 2 NH, (C 2 H 5) 2 NH, etc.); in the production of nylon (NH 2 -(CH 2) 6 -NH 2 - hexamethylenediamine); as a raw material for the production of dyes and plastics (aniline), as well as pesticides.
List of sources used:
- O.S. Gabrielyan et al. Chemistry. Grade 10. Profile level: textbook for general education institutions; Bustard, Moscow, 2005;
- “Chemistry Tutor” edited by A. S. Egorov; "Phoenix", Rostov-on-Don, 2006;
- G. E. Rudzitis, F. G. Feldman. Chemistry 10th grade. M., Education, 2001;
- https://www.calc.ru/Aminy-Svoystva-Aminov.html
- http://www.yaklass.ru/materiali?mode=lsntheme&themeid=144
- http://www.chemel.ru/2008-05-24-19-21-00/2008-06-01-16-50-05/193-2008-06-30-20-47-29.html
- http://cnit.ssau.ru/organics/chem5/n232.htm
Amins came into our lives completely unexpectedly. Until recently, these were toxic substances, a collision with which could lead to death. And now, a century and a half later, we actively use synthetic fibers, fabrics, building materials, and dyes based on amines. No, they did not become safer, people were simply able to “tame” them and subjugate them, deriving certain benefits for themselves. We'll talk about which one further.
Definition
For the qualitative and quantitative determination of aniline in solutions or compounds, a reaction is used, at the end of which a white precipitate in the form of 2,4,6-tribromoaniline falls to the bottom of the test tube.
Amines in nature
Amines are found everywhere in nature in the form of vitamins, hormones, and intermediate metabolic products; they are found both in the body of animals and in plants. In addition, the decay of living organisms also produces medium amines, which in a liquid state emit an unpleasant odor of herring brine. The “cadaveric poison” widely described in the literature appeared precisely thanks to the specific amber of amines.
For a long time, the substances we were considering were confused with ammonia because of their similar smell. But in the mid-nineteenth century, the French chemist Wurtz was able to synthesize methylamine and ethylamine and prove that when burned they release hydrocarbons. This was the fundamental difference between the mentioned compounds and ammonia.
Production of amines in industrial conditions
Since the nitrogen atom in amines is in the lowest oxidation state, the reduction of nitrogen-containing compounds is the simplest and most accessible way to obtain them. It is this that is widespread in industrial practice because of its cheapness.
The first method is the reduction of nitro compounds. The reaction during which aniline is formed is named by the scientist Zinin and was carried out for the first time in the mid-nineteenth century. The second method is to reduce amides using lithium aluminum hydride. Primary amines can also be recovered from nitriles. The third option is alkylation reactions, that is, the introduction of alkyl groups into ammonia molecules.
Application of amines

By themselves, in the form of pure substances, amines are rarely used. One of the rare examples is polyethylene polyamine (PEPA), which in domestic conditions facilitates the hardening of epoxy resin. Basically a primary, tertiary or secondary amine is an intermediate product in the production of various organic matter. The most popular is aniline. It is the basis of a large palette of aniline dyes. The color you get in the end depends directly on the selected raw material. Pure aniline produces a blue color, but a mixture of aniline, ortho- and para-toluidine will be red.
Aliphatic amines are needed to produce polyamides, such as nylon and others. They are used in mechanical engineering, as well as in the production of ropes, fabrics and films. In addition, aliphatic diisocyanates are used in the manufacture of polyurethanes. Due to their exceptional properties (lightness, strength, elasticity and the ability to attach to any surface), they are in demand in construction (foam, glue) and in the footwear industry (anti-slip soles).
Medicine is another area where amines are used. Chemistry helps synthesize antibiotics from the sulfonamide group from them, which are successfully used as second-line drugs, that is, backup. In case bacteria develop resistance to essential drugs.
Harmful effects on the human body
It is known that amines are very toxic substances. Any interaction with them can cause harm to health: inhalation of vapors, contact with open skin, or ingestion of compounds into the body. Death occurs from a lack of oxygen, since amines (in particular, aniline) bind to hemoglobin in the blood and prevent it from capturing oxygen molecules. Alarming symptoms are shortness of breath, blue discoloration of the nasolabial triangle and fingertips, tachypnea (rapid breathing), tachycardia, loss of consciousness.
If these substances get on bare areas of the body, you must quickly remove them with cotton wool previously soaked in alcohol. This must be done as carefully as possible so as not to increase the area of contamination. If symptoms of poisoning appear, you should definitely consult a doctor.
Aliphatic amines are poison for the nervous and cardiovascular systems. They can cause depression of liver function, liver dystrophy, and even bladder cancer.
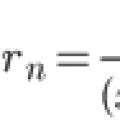 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A