When catalysts are used, the reaction route changes. The influence of a catalyst on the rate of chemical reactions
Substances that participate in reactions and increase its speed, remaining unchanged at the end of the reaction, are called catalysts.
The phenomenon of a change in reaction rate under the influence of such substances is called catalysis. Reactions that occur under the influence of catalysts are called catalytic.
In most cases, the effect of a catalyst is explained by the fact that it reduces the activation energy of a reaction. In the presence of a catalyst, the reaction proceeds through different intermediate stages than without it, and these stages are more energetically accessible. In other words, in the presence of a catalyst, other activated complexes arise, and their formation requires less energy than the formation of activated complexes that arise without a catalyst. Thus, the activation energy decreases sharply: some molecules, whose energy was insufficient for active collisions, now turn out to be active.
For a number of reactions, intermediates have been studied; as a rule, they are very active unstable products.
The mechanism of action of catalysts is associated with a decrease in the activation energy of the reaction due to the formation of intermediate compounds. Catalysis can be represented as follows:
A + K = A...K
A...K + B = AB + K,
where A...K is an intermediate activated compound.
Figure 13.5 - Image of the reaction path of a non-catalytic A + B → AB reaction (curve 1) and a homogeneous catalytic reaction (curve 2).
IN chemical industry catalysts are used very widely. Under the influence of catalysts, reactions can accelerate millions of times or more. In some cases, under the influence of catalysts, reactions can be excited that practically do not occur under given conditions without them.
Distinguish homogeneous and heterogeneous catalysis.
When homogeneous catalysis the catalyst and reactants form one phase (gas or solution). When heterogeneous catalysis the catalyst is in the system as an independent phase.
Examples of homogeneous catalysis:
1) oxidation of SO 2 + 1/2O 2 = SO 3 in the presence of NO; NO is easily oxidized to NO 2, and NO 2 already oxidizes SO 2;
2) decomposition of hydrogen peroxide in an aqueous solution into water and oxygen: ions Cr 2 O 2 = 7, WO 2-4, MoO 2-4, catalyzing the decomposition of hydrogen peroxide, form intermediate compounds with it, which further decompose with the release of oxygen.
Homogeneous catalysis is carried out through intermediate reactions with a catalyst, and as a result, one reaction with a high activation energy is replaced by several with lower activation energies and a higher rate:
CO + 1/2O 2 = CO 2 (catalyst - water vapor).
Heterogeneous catalysis is widely used in the chemical industry. Most of the products currently produced by this industry are obtained using heterogeneous catalysis. In heterogeneous catalysis, the reaction occurs on the surface of the catalyst. It follows that the activity of the catalyst depends on the size and properties of its surface. In order to have a large (“developed”) surface, the catalyst must have a porous structure or be in a highly crushed (highly dispersed) state. At practical application the catalyst is usually applied to a carrier having a porous structure (pumice, asbestos, etc.).
As in the case of homogeneous catalysis, in heterogeneous catalysis the reaction proceeds through active intermediates. But here these compounds are surface compounds of the catalyst with the reacting substances. Passing through a series of stages involving these intermediates, the reaction ends with the formation of final products, and the catalyst is not consumed as a result.
All catalytic heterogeneous reactions include the stages of adsorption and desorption.
The catalytic effect of the surface is reduced to two factors: an increase in the concentration at the interface and the activation of adsorbed molecules.
Examples of heterogeneous catalysis:
2H 2 O = 2H 2 O + O 2 (catalyst – MnO 2 ,) ;
H 2 + 1/2 O 2 = H 2 O (catalyst - platinum).
Catalysis plays a very important role in biological systems. Most chemical reactions occurring in the digestive system, in the blood and in the cells of animals and humans are catalytic reactions. Catalysts, called enzymes in this case, are simple or complex proteins. Thus, saliva contains the enzyme ptyalin, which catalyzes the conversion of starch into sugar. An enzyme found in the stomach, pepsin, catalyzes the breakdown of proteins. There are about 30,000 different enzymes in the human body: each of them serves as an effective catalyst for the corresponding reaction.
Catalysts are substances that can accelerate a chemical reaction, without the catalysts themselves being consumed in the chemical reaction. It has been established that catalysts change the mechanism of a chemical reaction. In this case, other, new transition states arise, characterized by a lower energy barrier height. Thus, under the influence of the catalyst, it decreases
activation energy of the process (Fig. 3). Entering into various types of interactions with intermediate particles, the catalysts remain in unchanged quantities at the end of the reaction. Catalysts act only on thermodynamically allowed reactions. The catalyst cannot cause a reaction because does not affect its driving forces. The catalyst does not affect the chemical equilibrium constant, because equally reduces the activation energy of both forward and reverse reactions.
Fig.3 Energy diagram of the reaction A + B = AB a) without a catalyst and b) in the presence of a catalyst. Ea is the activation energy of a non-catalytic reaction; Ea 1 and Ea 2 - activation energy of the catalytic reaction; AA is an intermediate reactive compound of the catalyst with one of the reagents; A...K, AK...B – activated complexes of the catalytic reaction; A…B - activated complex of a non-catalytic reaction; ∆E cat. – decrease in activation energy under the influence of the catalyst.
There are homogeneous and heterogeneous catalysis. In the first case, the catalyst is in the same phase with the reagents, and in the second, the catalyst is solid, on the surface of which a chemical reaction between reagents takes place.
Chemical equilibrium
Chemical reactions are usually divided into reversible and irreversible. Irreversible chemical reactions proceed until at least one of the starting substances is completely consumed, i.e. The reaction products either do not interact with each other at all, or form substances different from the original ones. There are very few such reactions. For example:
2KClO 3 (tv) = 2KCl (tv) + 3O 2 (g)
In electrolyte solutions, reactions that occur with the formation of precipitation, gases and weak electrolytes (water, complex compounds) are considered practically irreversible.
Most chemical reactions are reversible, i.e. they go both forward and backward. This becomes possible when the activation energies of the direct and reverse processes differ slightly from each other, and the reaction products are able to transform into the starting substances. For example, the HI synthesis reaction is a typically reversible reaction:
H 2(g) +I 2(g) ⇄ 2HI (g)
The law of mass action (expression of the reaction rate) for direct and reverse processes, respectively, will have the form: = ∙ ;  = 2
= 2
At some point in time, a state occurs when the rates of forward and reverse reactions become equal = (Fig. 4).

Fig. 4 Change in the rates of forward ( and reverse ( reactions) over time t
This state is called chemical equilibrium. It is dynamic (moving) in nature and can shift in one direction or another depending on changes in external conditions. Starting from the moment of equilibrium, under constant external conditions, the concentrations of the starting substances and reaction products do not change over time. The concentrations of reagents corresponding to the equilibrium state are called equilibrium. To determine the equilibrium concentration of a reagent, it is necessary to subtract from its initial concentration the amount of substance that has reacted by the time the equilibrium state occurs: WITH equal = C ref. - WITH pro-reacter. The number of reagents that entered into the reaction and formed from them at the time of equilibrium of the products is proportional to the stoichiometric coefficients in the reaction equation.
A state of equilibrium under constant external conditions can exist indefinitely. In a state of balance
∙ = [ 2 , whence / [= 2 / ∙ .
At a constant temperature, the rate constants of the forward and reverse processes are constant values.
The ratio of two constants is also the value of the constant K= / and is called chemical equilibrium constant. It can be expressed
either through the concentrations of the reactants = , or through their partial pressures  , if the reaction occurs with the participation of gases.
, if the reaction occurs with the participation of gases.
In the general case, for the reaction aA+bB+ …⇄cC+dD+ …the chemical equilibrium constant is equal to the ratio of the product of the concentrations of the reaction products to the product of the concentrations of the starting substances in powers equal to their stoichiometric coefficients.
The chemical equilibrium constant does not depend on the path of the process and determines the depth of its occurrence by the time the equilibrium state is reached. The larger this value, the greater the degree of conversion of reactants into products.
The chemical equilibrium constant, as well as the reaction rate constants, are functions only of the temperature and nature of the reacting substances and do not depend on their concentration.
For heterogeneous processes, the concentration of solids is not included in the expression of the reaction rate and chemical equilibrium constant, because the reaction occurs on the surface of the solid phase, the concentration of which remains constant over time. For example, for a reaction:
FeO (s) + CO (g) ⇄ Fe (s) + CO 2 (g)
the expression for the equilibrium constant will be:
K p and K c are related by the relation K p = K c (RT) ∆ n, wheren=n cont. -n raw materials – change in the number of moles gaseous substances during the reaction. For this reaction, K p = K c, since n of gaseous substances is zero.
In grades IX-X high school continue to formulate concepts about the rate of chemical reactions, the influence of various factors on the rate of chemical transformations, expand and deepen knowledge about catalysis and catalysts, and give some ideas about the mechanism of catalytic phenomena.
In the topic " Alkali metals", demonstrating experiments such as the interaction of sodium with water and hydrochloric acid, the interaction of potassium and sodium with water, the teacher emphasizes that some of these reactions occur faster than others under the same conditions. For example, sodium reacts more vigorously with hydrochloric acid than with water; Potassium reacts more vigorously with water than sodium. After combustion experiments in chlorine with sodium, copper, antimony, hydrogen, organic matter You can ask questions: “Why was antimony powder used for combustion in chlorine, and not pieces? Why does a bundle of thin copper wire burn in chlorine, but a thick wire does not?” In these cases, the difference in the interaction of substances is explained either by the nature of the substances themselves and the structure of atoms, or by a different contact surface.
In the same topic, when introducing students to the properties of hydrochloric acid, it is useful to find out why reactions between this acid and metals (zinc, magnesium) accelerate over time. The acceleration depends, in particular, on the fact that these reactions release a large number of heat, and with heating of substances the rate of interaction increases.
Using the example of the reaction between aluminum and iodine, it is worth recalling what a catalyst is and showing that water can be a catalyst. A mixture of iodine and aluminum powders is poured onto an asbestos mesh in a heap and a few drops of water are added. The interaction of substances under the influence of water accelerates, and a flame breaks out. The teacher draws attention to the fact that in the mixture that was not poured out of the porcelain cup onto the mesh, a flash did not occur, but it can occur after some time and without water.
It should be noted that water not only accelerates the interaction of aluminum with iodine, but also plays a catalytic role in many chemical processes. The catalytic effect of water during the combustion of various gases used in technology is very important.
When considering the properties of hydrogen peroxide, it is indicated that hydrogen peroxide is a very fragile substance. When stored in glass containers, it slowly decomposes, releasing heat:
2H 2 O 2 = 2H 2 O 4 + O 2 + 46 kcal
The teacher asks students to list the conditions that accelerate the decomposition of hydrogen peroxide. They can
indicate in this case: 1) heating, 2) the action of catalysts, 3) increasing the concentration of the solution. It can be added that the decomposition of hydrogen peroxide also occurs faster in the light; this can be confirmed by experience in extracurricular activities. Pour hydrogen peroxide into two flasks secured in stands and close them with stoppers with gas outlet tubes. Place the tubes under overturned cylinders or test tubes filled with water and lowered into a wide vessel with water. Wrap one of the flasks in black paper. Place the devices in a sunny window or illuminate them with an electric lamp at 75-100 V. Experiment will show the rapid decomposition of hydrogen peroxide under the influence of light.
Then, during the lesson, students independently study the change in the rate of decomposition of hydrogen peroxide under the influence of catalysts. For work, you are given a 3-5% solution of hydrogen peroxide, manganese dioxide, concentrated hydrochloric acid, a splinter, a funnel, filter paper, and several test tubes.
Tasks: 1) Check whether hydrogen peroxide is decomposing in the solution that was issued? 2) Using manganese dioxide, accelerate the decomposition reaction of hydrogen peroxide. 3) Prove that manganese dioxide did not change chemically as a result of the reaction * 4) Prove that manganese dioxide, already used as a catalyst, can again accelerate the decomposition of hydrogen peroxide.
* (Test with hydrochloric acid when heated.)
After graduation independent work the teacher shows that different catalysts can be used to speed up the same chemical reaction, that decomposition inorganic matter(hydrogen peroxide) is accelerated by organic catalysts - enzymes. A 3% solution of hydrogen peroxide is poured into a small beaker, then a small piece of raw meat is placed in it. Oxygen is intensively released from the solution, since the blood and tissues of animals contain the enzyme catalase. It should be emphasized that enzymes are excellent natural reaction accelerators. One of the important tasks of future chemistry is the artificial production and industrial use of catalysts that will resemble enzymes in their composition and catalytic properties.
To explain why the decomposition of hydrogen peroxide is faster when stored in glass containers, an experiment is carried out. A solution of hydrogen peroxide is poured into three test tubes, a solution of sulfuric acid is added to one of them, caustic soda is added to the other, and the third is left for comparison (control solution). All three solutions are heated (not to boiling). Oxygen will be strongly released from a test tube with solutions of hydrogen peroxide and sodium hydroxide, less strongly - from a test tube with a control solution. In the presence of sulfuric acid (hydrogen ions), hydrogen peroxide does not decompose. OH ions catalyze the process of decomposition of hydrogen peroxide, therefore, in a glass container, the walls of which release hydroxyl ions into the solution, hydrogen peroxide easily decomposes.
Consolidation and development of knowledge about the rate of chemical reactions continues. By passing a mixture of sulfur dioxide and oxygen through a heated glass tube without a catalyst, the teacher shows that the formation sulfuric anhydride under these conditions it is not noticeable, and asks students how the interaction of gases can be accelerated. During the conversation, it turns out that such methods of accelerating reactions as increasing the concentrations of reagents, increasing the temperature, without the use of a catalyst, do not give the necessary results. The oxidation reaction of sulfur dioxide to sulfur dioxide is reversible:
2SO 2 + O 2 ↔ 2SO 3 + Q,
and an increase in temperature accelerates the decomposition of sulfuric anhydride to a greater extent than its formation.
They check whether iron oxide will be a catalyst for the oxidation reaction of sulfur dioxide. When demonstrating the contact oxidation of sulfur dioxide into sulfuric anhydride in the presence of iron oxide, the formation of sulfuric anhydride is observed, fuming in air. It is then established that the iron oxide has not changed chemically as a result of the reaction. To do this, repeat the experiment of contact oxidation of sulfur dioxide into sulfuric anhydride with the same portion of iron oxide. It is further noted that various catalysts can be used to accelerate the oxidation of sulfur dioxide. In addition to iron oxide, platinum was used in the chemical industry, and now vanadium pentoxide V 2 O 5 * is used.
* (The vanadium catalyst currently used has a complex composition (see: D. A. Epshtein. Chemistry teacher about chemical technology, M., Publishing House of the Academy of Sciences of the RSFSR, 1961).)
It is also important to emphasize the property of the catalyst, accelerating the reaction, without affecting its reversibility: the oxidation reaction of sulfur dioxide into sulfur dioxide remains reversible even if a catalyst is used.
When studying the contact method for the production of sulfuric acid, it is necessary to consider the use of a catalyst in industry. Without a catalyst, the rapid production of large quantities of sulfuric anhydride would be impossible, but its use causes some Additional requirements to the process conditions. The fact is that impurities in the reactants negatively affect the catalyst. Arsenic trioxide has a negative effect on the vanadium catalyst, as they say, “poisons” it. Therefore, careful purification of the reacting gases from impurities is necessary.
If students have a question about why the catalyst is poisoned, the teacher first explains its action using the theory of formation of intermediate compounds, and then considers the poisonous effect of impurities.
Acceleration of reactions with the help of a catalyst occurs due to the fact that it forms weak compounds with the starting substances, and then is released again in a free form. These reactions proceed much faster than the reaction between sulfur dioxide and oxygen. If the mixture of gases contains impurities that enter into irreversible reactions with the catalyst, then its poisoning occurs. Despite careful purification of gases, the activity of catalysts used in the production of sulfuric acid decreases over time. Its “aging” is caused not only by gradual poisoning, but also by prolonged heating and mechanical destruction, which change the state of the catalyst surface. Not the entire surface of the catalyst participates in the catalyzed reaction, but only its trimmed sections - active centers, and the number of these centers decreases with “aging”.
The previous section examined how, in the light of the theory of atomic structure, the effect of energy on the initiation of a chemical reaction should be explained to students. This will make it possible to solve the question of why chemical reactions accelerate when heated. Students know that as the temperature in substances increases, the number of active molecules increases, the speed of movement of molecules and the number of their meetings per unit time increases. In the atoms of active molecules, electrons are moved to higher energy levels; such molecules are unstable and can more easily react with molecules of other substances.
The theory of electrolytic dissociation explains why reactions between solutions of acids, salts and bases occur almost instantly. Solutions of these substances already contain active particles - oppositely charged ions. Therefore, reactions between aqueous solutions of acids, salts and bases proceed very quickly and differ significantly from reactions between the same substances, but taken in dry form.
Starting a lesson on the topic “Rate of a chemical reaction,” the teacher reminds that chemical reactions can occur at different speeds; studying the conditions that affect it is of great practical importance.
How can you measure the rate of a chemical reaction?
Students already know that the speed of a chemical transformation can be judged by the amount of a substance that reacted or was produced in a certain time, that the speed of mechanical movement is measured by the path that a body travels per unit time; To calculate this speed, use the formula
where v is speed, S is path and t is time.
Taking this into account, students write by analogy a formula for calculating the rate of a chemical reaction
where m is the amount of substance that entered into the reaction or was obtained as a result of it during time t.
Consider what is the disadvantage of this formula. It turns out that when using it, the calculated reaction rate will be different even for two portions of the same substance taken under the same conditions.
Let us assume that 15 g of a substance decomposes in a vessel every second. It turns out that when a partition is introduced into this vessel, which will divide the substance in it into two parts in a ratio of 1:2, in the first (smaller) part the reaction will proceed at a rate of 5 g/sec, and in the second - 10 g/sec.
In order for the calculated rate to characterize the reaction itself, and not how much of the starting substance is taken, it is necessary to take into account the change in the mass of the reactant per volume, i.e., the change in the concentration of the reactant. Therefore, the rate of a chemical reaction can be calculated using the formula:
v=c 0 -c t /t
where c 0 is the initial concentration of any of the reacting substances, c t is the concentration of the same substance after t seconds. When calculating speed, concentration is usually expressed in moles per liter and time in seconds.
This lesson focuses on the most important ways to speed up chemical reactions. For this purpose, a laboratory experiment is carried out showing that the rate of a chemical reaction depends on the concentration of the reacting substances.
For the experiment, the following equipment is used, placed on the student tables: 1) a stand with three test tubes, one of which contains a crystal of sodium iodide or potassium iodide (the size of 2 - 3 pin heads), the other contains a solution of ferric chloride, and the third - empty; 2) a flask or glass of water; 3) two identical glass tubes; 4) glass rod.
The teacher invites students to prepare for the experiment: 1) add water to sodium iodide to form 1/2 test tube of solution and mix the liquid with a stick, 2) pour 1/3 of the resulting solution into another test tube, 3) add to the solution poured into another test tube test tube with a solution of water so that the volumes of solutions of sodium iodide (or potassium iodide) in the test tubes are the same.
The teacher asks questions to check students' understanding of the directions:
1) How many times is the sodium iodide solution diluted in the second test tube?
2) How many times is the salt concentration in the first test tube greater than in the second?
It is noted that the concentration of one of the solutions is twice the concentration of the other. After this, in two prepared solutions, ferric chloride is reacted with sodium iodide, which releases free iodine:
2NaI + 2FeCl 3 = 2NaCl + 2FeCl 2 + I 2,
2I - + 2Fe 3+ = 2Fe 2+ + I 2.
Students decide in which test tube the rate of interaction of salts is greater and by what criteria this can be judged. The assumption is tested experimentally.
First, equal amounts of starch paste (1-2 ml) are poured into both test tubes with solutions of sodium iodide (or potassium iodide), and then, after mixing, a few drops of a 5-10% solution of ferric chloride. It is advisable to pour the ferric chloride solution into both test tubes at the same time. The blue color appears more likely in a test tube with a solution of higher concentration. In the test tube where the concentration of the solution is higher, iodine ions are more likely to meet with ferric ions, and therefore interact with them more often - the reaction proceeds faster.
The teacher shows the combustion of sulfur in the air and asks students how this reaction can be accelerated. Students suggest placing burning sulfur in oxygen and perform this experiment. Based on the analysis of the experiments, a general conclusion is drawn: the rate of a chemical reaction depends on the concentration of the reacting substances (the number of ions or molecules per unit volume).
We move on to the question of the influence of the surface of reacting substances on the rate of chemical reaction. Students recall reactions involving mixing and grinding of reacting substances: grinding a mixture of ammonia with slaked lime, the interaction of small pieces of marble or zinc with hydrochloric acid, the combustion of pulverized fuel in nozzles, the use of crushed ores in the smelting of metals and sulfur pyrites in the production of sulfuric acid. The conditions for firing pyrites in the production of sulfuric acid are discussed in more detail. To produce sulfur dioxide, crushed pyrite is used, since it burns faster than pyrite taken in large pieces. The combustion of dusted pyrite occurs especially quickly if it is ejected with a stream of air from a nozzle, as well as when it is burned in a fluidized bed, when the entire surface of the pieces of pyrite comes into contact with air.
It must be taken into account that chemical reactions with highly crushed flammable substances can be accompanied by an explosion. There have been, for example, sugar dust explosions at sugar producing factories.
They conclude that the more crushed a solid is, the faster the rate of the chemical reaction in which it participates.
Then the effect of temperature on the rate of a chemical reaction is analyzed. The same amount of sulfuric acid solution is poured into a test tube with 1/4 of the hyposulfite solution; In parallel with this experiment, heated solutions of hyposulfite and sulfuric acid are drained:
Na 2 S 2 O 3 + H 2 SO 4 = Na 2 SO 4 + H 2 O + SO 2 + S↓
The time until the solutions become cloudy is noted. The teacher says that when the temperature increases by 10° C, the rate of most reactions increases by 2-3 times.
Based on the acquired knowledge, students are given the opportunity to explain the acceleration of chemical reactions when substances are heated.
In this lesson there is no need to demonstrate experience in the catalytic effects of substances, since students became familiar with it using examples of the reaction of the decomposition of hydrogen peroxide and the oxidation of sulfur dioxide. They list the catalytic reactions known to them and give definitions of catalysis and catalyst.
To consolidate knowledge in this lesson, the following questions are asked:
- What determines the rate of a chemical reaction? Give examples.
- Under what conditions does the rate of a chemical reaction increase?
- How, in the light of the theory of electrolytic dissociation, can one explain that the evolution of hydrogen when zinc reacts with acetic acid occurs much more slowly than when zinc reacts with hydrochloric acid?
- In what ways can you speed up the reaction between zinc and hydrochloric acid?
- Why does a splinter smoldering in air flare up in oxygen?
- You have been given two test tubes in which calcium carbonate slowly reacts with hydrochloric acid. Try to speed up the chemical reaction in each test tube using different techniques.
- Why does the rate of a chemical reaction increase with increasing temperature?
- What methods of accelerating chemical reactions are used in the production of sulfuric acid?
- List which chemical reactions known to you are accelerated by catalysts.
When studying the reaction of ammonia synthesis, students again encounter the use of a catalyst, and, along with consolidating previously acquired information about catalysis and catalysts, this knowledge can be somewhat developed.
The teacher draws attention to the fact that both reactions - the synthesis of ammonia and its decomposition into nitrogen and hydrogen - occur in the presence of the same catalyst - reduced iron, which accelerates both the forward and reverse reactions to the same extent. Therefore, the catalyst does not shift the chemical equilibrium, but only contributes to a faster achievement of this state. To check their understanding of this provision, the teacher asks them questions:
- Is it possible to produce ammonia in production from a mixture of nitrogen and hydrogen under high pressure and heating, but without a catalyst? Why?
- The ammonia synthesis reaction is accelerated by heat and a catalyst. What is the difference in the influence of these conditions on chemical equilibrium?
Introducing students to the synthesis of ammonia in production, the teacher points out that the catalyst quickly loses its activity if the gases (hydrogen and nitrogen) are not first freed from impurities. In this process, oxygen, water vapor, carbon monoxide, hydrogen sulfide and other sulfur compounds have a poisonous effect.
As in the case of the catalytic oxidation of sulfur dioxide into trioxide, during the synthesis of ammonia the catalyst exerts its accelerating effect only within certain temperature limits. At temperatures above 600° C, reduced iron reduces its catalytic activity.
Using the example of ammonia synthesis, we can consider the mechanism of action of the catalyst. It is noted that iron nitride is formed on the surface of the iron catalyst:
Hydrogen reacts with nitride to produce ammonia:
FeN 2 + 3H 2 → Fe + 2NH 3.
Then the process is repeated.
The reactions of iron nitride formation and its interaction with hydrogen proceed very quickly.
When studying the reactions of ammonia oxidation, after demonstrating experiments on the combustion of ammonia in oxygen and the catalytic oxidation of ammonia, students’ attention is drawn to the fact that the starting substances in these two cases were taken the same, but depending on the conditions (use of a catalyst), different products are obtained .
Ammonia oxidation can occur with the formation of different substances according to the equations:
4NH 3 + 3O 2 = 2N 2 + 6H 2 O;
4NH 3 + 4O 2 = 2N 2 O + 6H 2 O;
4NH 3 + 5O 2 = 4NO + 6H 2 O.
The catalyst, platinum, accelerates only the last of these reactions. Therefore, using a catalyst, it is possible to direct the interaction of ammonia and oxygen in the desired direction. This finds application in chemical production in the production of nitric acid.
Forming the concept of chemical production in grade IX provides great opportunities for introducing students to the practical control of the rate of chemical reactions in chemical plants.
Based on the generalization of knowledge about previously studied production (hydrochloric, sulfuric, nitric acids, ammonia), the teacher forms in students the concept of the best conditions for conducting chemical reactions in production: the use of optimal temperatures, increasing the concentration of reacting substances, increasing the contact surface of reacting substances, and the use of catalysts. After this, in order to identify the circumstances limiting the use of each condition, students are asked the question: “Is it possible to increase the temperature indefinitely to accelerate chemical reactions in production?” They find out that strong heating can shift the chemical equilibrium in an undesirable direction, and in the case of using a catalyst, reduce its activity. Taking this into account, not maximum, but optimal temperatures are used in production.
Other conditions for conducting chemical reactions in production are analyzed in the same way.
The study of new factual material in chemistry in grades IX-X is used to further consolidate knowledge about the rate of chemical reactions.
When studying the properties of white phosphorus, the teacher says that the glow of white phosphorus in the dark indicates its slow oxidation in air. Next, we consider under what conditions the oxidation of white phosphorus can be accelerated. Heating, crushing phosphorus, and the use of oxygen actually accelerate the oxidation of phosphorus, causing it to flare.
Students use knowledge about ways to accelerate chemical processes to predict the conditions for the formation of superphosphate. They say that the reaction between tertiary calcium phosphate and sulfuric acid can be accelerated by heating, grinding the calcium phosphate, stirring, and increasing the concentration of sulfuric acid. The teacher, summarizing what has been said, adds that in
In this production, heating is indeed used, but for this they use the heat released during the reaction itself, when crushed tertiary calcium phosphate is thoroughly mixed with sulfuric acid.
As students study organic substances, they encounter many processes that involve catalysts, for example, the production of aviation gasoline, rubber, and aromatic hydrocarbons.
We can consider the role of sulfuric acid in the hydration of ethylene. In the presence of sulfuric acid, instead of the slow reaction of adding water to ethylene (C 2 H 4 + H 2 O → C 2 H 5 OH), the following processes occur quickly one after another: 1) sulfuric acid combines with ethylene to form ethyl sulfur ether:
2) ethyl sulfur ether undergoes saponification to form ethyl alcohol and sulfuric acid.
After distilling off the alcohol, sulfuric acid appears in the same amount, but it took part in the formation of the intermediate product. Students examine other examples of the catalytic action of sulfuric acid (formation of ethylene and ethyl ether from ethyl alcohol) independently when doing their homework.
The same substances with the same catalyst, but at different temperatures, react to form different products. This should be emphasized when familiarizing yourself with the properties of alcohols.
The interaction of carbon monoxide with hydrogen shows that using different catalysts, different organic products can be obtained from the same substances. This interaction can lead to the formation of methyl alcohol, hydrocarbons or higher alcohols. The desired direction of interaction of substances is achieved through the use of a catalyst that accelerates the corresponding reaction, but does not have a significant effect on others. To speed up the reaction of methyl alcohol formation, a mixture of chromium oxides and zinc oxide is used as a catalyst.
After studying hydrocarbons and oxygen-containing organic compounds To generalize knowledge, students are offered a task for independent work in class or at home: select from such and such a section of the textbook all cases of catalytic reactions, and each student is given only such a part of the textbook material that he can view in the allotted time.
Taking apart industrial methods obtaining organic substances, it is useful to draw students’ attention to the fact that to control the rates of chemical reactions, the same techniques are used that are used in the production of inorganic
38. The influence of a catalyst on the rate of chemical reactions. Reasons for the influence of the catalyst.
Substances that are not consumed as a result of a reaction, but affect its rate, are called catalysts. Catalysts that reduce the rate of a reaction are called inhibitors. The effect that catalysts have on chemical reactions is called catalysis . The essence of catalysis is that in the presence of a catalyst, the path along which the overall reaction proceeds changes, other transition states with different activation energies are formed, and therefore the rate of the chemical reaction also changes. There are homogeneous and heterogeneous catalysis. In heterogeneous catalysis, the reaction occurs on the surface of the catalyst. It follows that the activity of the catalyst depends on the size and properties of its surface. In order to have a large surface area, the catalyst must have a porous structure or be in a highly fragmented state. Catalysts are distinguished by selectivity: they act selectively on processes, directing them in a certain direction. Negative catalysis is used to slow down corrosion.
39. Reversible processes. Chemical balance. Equilibrium constant.
Reactions that proceed in only one direction and end with the complete conversion of the initial reactants into the final substances are called irreversible. 2KClO 3 = 2KCl + 3O 2 . Reversible are reactions that occur simultaneously in two mutually opposite directions. 3H 2 + N 2 ⇆ 2NH 3
Reversible reactions do not proceed to completion: none of the reactants are completely consumed. Reversible processes: at first, when mixing the starting substances, the rate of the forward reaction is high, and the rate of the reverse reaction is zero. As the reaction proceeds, the starting materials are consumed and their concentrations fall, resulting in a decrease in the reaction rate. At the same time, reaction products appear, whose concentration increases, and, accordingly, the rate of the reverse reaction increases. When the rates of the forward and reverse reactions become equal, chemical equilibrium occurs. It is called dynamic equilibrium, since forward and reverse reactions occur, but due to the same speeds, changes in the system are not noticeable. A quantitative characteristic of chemical equilibrium is a value called the chemical equilibrium constant. At equilibrium, the rates of the forward and reverse reactions are equal, while constant concentrations of the starting substances and reaction products, called equilibrium concentrations, are established in the system. For 2CO + O 2 = 2CO 2 the equilibrium constant can be calculated using the equation: The numerical value of the equilibrium constant, to a first approximation, characterizes the yield of a given reaction. The yield of a reaction is the ratio of the amount of substance obtained to the amount that would be obtained if the reaction proceeded to completion. K>>1 reaction yield is high, K<10-6). В случае гетерогенных реакций в выражение константы равновесия входят концентрации только тех веществ, которые находятся в наиболее подвижной фазе. Катализатор не влияет на константу равновесия. Он может только ускорить наступление равновесия. K=e^(-ΔG/RT).
40. The influence of various factors on the displacement of equilibrium. Le Chatelier's principle.
If the system is in equilibrium, then it will remain in it as long as external conditions remain constant. The process of changing any conditions affecting equilibrium is called a shift in equilibrium.
Le's principle: If on syst. find. in balance to exert an external influence, then the system of change. in such a way as to compensate for this impact.
Consequences: 1) With increasing temperature. balance displacement in favor of an endothermic reaction.
2) As pressure increases, the equilibrium shifts. towards a smaller volume (or a smaller number of moles)
3) With an increase in the concentration of one of the starting substances, the equilibrium shifts towards an increase in the concentration of reaction products, and vice versa.
Why do catalysts increase the rate of a chemical reaction? It turns out that they act in full accordance with popular wisdom: “A smart person won’t climb a mountain, a smart person will walk around a mountain.” In order for substances to begin to interact, their particles (molecules, atoms, ions) need to be given a certain energy, called activation energy (Fig. 13, a). Catalysts lower this energy by combining with one of the reacting substances and moving it along an “energy mountain” to meet another substance with less energy. Therefore, in the presence of a catalyst, chemical reactions not only proceed faster, but also at a lower temperature, which reduces the cost of production processes.
Rice. 13.
Energy diagrams of catalytic reactions using a conventional (a) and selective (b) catalyst
And not only. The use of catalysts can lead to the same substances reacting differently, i.e., with the formation of different products (Fig. 13, b). For example, ammonia is oxidized by oxygen to nitrogen and water, and in the presence of a catalyst - to nitrogen oxide (II) and water (write down the reaction equations and consider the processes of oxidation and reduction).
The process of changing the rate of a chemical reaction or the path along which it occurs is called catalysis. Like reactions, there are homogeneous and heterogeneous types of catalysis. When enzymes are used, catalysis is called enzymatic. This type of catalysis has been known to man since ancient times. Thanks to the enzymatic breakdown of organic substances, man learned to bake bread, brew beer, and make wine and cheese (Fig. 14).

Rice. 14.
Since ancient times, man has used catalysis, which occurs when baking bread, brewing beer, making wine, making cheese
The most commonly known enzymes in everyday life are those found in washing powders. They allow you to rid your laundry of stains and unpleasant odors during washing.
Let's take a closer look at catalysts using a chemical experiment.
Hydrogen peroxide (in everyday life it is often called hydrogen peroxide) is a necessary drug in any home medicine cabinet (Fig. 15).

Rice. 15.
Hydrogen peroxide solution
The expiration date must be indicated on the packaging of this drug, since it decomposes during storage:
![]()
However, under normal conditions, this process proceeds so slowly that we do not notice the release of oxygen, and only by opening a bottle in which hydrogen peroxide has been stored for a long time can we notice how a little gas is released from it. How to speed up this process? Let's conduct a laboratory experiment.
Laboratory experiment No. 9 Decomposition of hydrogen peroxide using manganese (IV) oxide
Laboratory experiment No. 10
Detection of catalase in food products
Catalysts not only make production processes more economical, but also make a significant contribution to environmental protection. Thus, modern passenger cars are equipped with a catalytic device, inside of which there are ceramic cellular catalyst carriers (platinum and rhodium). Passing through them, harmful substances (carbon oxides, nitrogen oxides, unburned gasoline) are converted into carbon dioxide, nitrogen and water (Fig. 16).

Rice. 16.
A car's catalytic converter that converts nitrogen oxides from its exhaust gases into harmless nitrogen.
However, for chemical reactions, not only catalysts that speed up the reaction are important, but also substances that can slow them down. Such substances are called inhibitors. The best known are metal corrosion inhibitors.
Laboratory experiment No. 11
Inhibition of the interaction of acids with metals by methenamine
In the vocabulary of an ordinary person there are often words that are borrowed from chemistry. For example, antioxidants, or antioxidants. What are substances called antioxidants? You've probably noticed that if you store butter for a long time, it changes color, taste, and acquires an unpleasant odor - it oxidizes in air. To prevent food products from spoiling, antioxidants are added to them. They also play an important role in maintaining human health, because unwanted oxidation processes also occur in the body, as a result of which a person gets sick, gets tired and ages faster. The human body receives antioxidants by eating foods containing, for example, carotene (vitamin A) and vitamin E (Fig. 17).

Rice. 17.
Antioxidants: a - β-carotene; b - vitamin E
So, the rate of a chemical reaction can be controlled using catalysts and inhibitors, changes in temperature, concentration of reacting substances, pressure (for homogeneous gas reactions), and contact area of reacting substances (for heterogeneous processes). And of course, the rate of chemical reactions depends on the nature of the reactants.
New words and concepts
- Catalysts.
- Enzymes.
- Catalysis (homogeneous, heterogeneous, enzymatic).
- Inhibitors.
- Antioxidants.
Tasks for independent work
- What are catalysts? What role do they play in chemical reactions? Why do catalysts speed up chemical reactions?
- What role did enzymatic catalysis play in the history of human civilization?
- Prepare a report on the role of catalysts in modern production.
- Prepare a report on the role of inhibitors in modern production.
- Prepare a report on the role of antioxidants in medicine and the food industry.
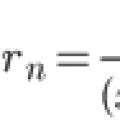 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A