Genetic series for sulfur. Genetic relationship between the main classes of inorganic substances
Instructions for students correspondence course“General chemistry for grade 12” 1. Category of students: the materials of this presentation are provided to the student for self-study topics “Substances and their properties”, from the course general chemistry 12th grade. 2. Course content: includes 5 presentations of topics. Each educational topic contains a clear structure educational material on a specific topic, the last slide is a control test - tasks for self-control. 3. Duration of training for this course: from one week to two months (determined individually). 4. Knowledge control: the student provides a report on completion test tasks– a sheet with options for assignments, indicating the topic. 5. Evaluation of the result: “3” - 50% of tasks completed, “4” - 75%, “5”% of tasks. 6. Learning result: pass (fail) of the topic studied.


Reaction equations: 1. 2Cu + o 2 2CuO copper (II) oxide 2. CuO + 2 HCl CuCl 2 + H 2 O copper (II) chloride 3. CuCl NaOH Cu(OH) Na Cl copper (II) hydroxide 4. Cu (OH) 2 + H 2 SO 4 CuSO 4 + 2H 2 O copper (II) sulfate



Genetic series organic compounds. If the basis of the genetic series is not organic chemistry are substances formed by one chemical element, then the basis of the genetic series in organic chemistry consists of substances with the same number of carbon atoms in the molecule.


Reaction scheme: Each number above the arrow corresponds to a specific reaction equation: ethanal ethanol ethene ethane chloroethane ethine Acetic (ethanoic) acid

Reaction equations: 1. C 2 H 5 Cl + H 2 O C 2 H 5 OH + HCl 2. C 2 H 5 OH + O CH 3 CH O + H 2 O 3. CH 3 CH O + H 2 C 2 H 5 OH 4. C 2 H 5 OH + HCl C 2 H 5 Cl + H 2 O 5. C 2 H 5 Cl C 2 H 4 + HCl 6. C 2 H 4 C 2 H 2 + H 2 7. C 2 H 2 + H 2 O CH 3 CH O 8. CH 3 CH O + Ag 2 O CH 3 COOH + Ag

First, we present our information about the classification of substances in the form of a diagram (Scheme 1).
Scheme 1
Classification of inorganic substances
Knowing the classes simple substances, two genetic series can be compiled: a genetic series of metals and a genetic series of non-metals.
There are two varieties of the genetic series of metals.
1. Genetic series of metals to which alkali corresponds as a hydroxide. IN general view such a series can be represented by the following chain of transformations:
For example, the genetic series of calcium:
Ca → CaO → Ca(OH) 2 → Ca 3 (PO 4) 2.
2. Genetic series of metals that correspond to an insoluble base. This series is richer in genetic connections, since it more fully reflects the idea of mutual transformations (direct and reverse). In general, such a series can be represented by the following chain of transformations:
metal → basic oxide → salt →
→ base → basic oxide → metal.
For example, the genetic series of copper:
Cu → CuO → CuCl 2 → Cu(OH) 2 → CuO → Cu.
Here, too, two varieties can be distinguished.
1. The genetic series of nonmetals, to which a soluble acid corresponds as a hydroxide, can be reflected in the form of the following chain of transformations:
non-metal → acidic oxide → acid → salt.
For example, the genetic series of phosphorus:
P → P 2 O 5 → H 3 PO 4 → Ca 3 (PO 4) 2.
2. The genetic series of nonmetals, which correspond to an insoluble acid, can be represented using the following chain of transformations:
nonmetal → acid oxide → salt →
→ acid → acid oxide → non-metal.
Since of the acids we have studied, only silicic acid is insoluble, as an example of the last genetic series, consider the genetic series of silicon:
Si → SiO 2 → Na 2 SiO 3 → H 2 SiO 3 → SiO 2 → Si.
Key words and phrases
- Genetic connection.
- Genetic series of metals and its varieties.
- Genetic series of nonmetals and its varieties.
Work with computer
- Refer to the electronic application. Study the lesson material and complete the assigned tasks.
- Find email addresses on the Internet that can serve as additional sources that reveal the content of keywords and phrases in the paragraph. Offer your help to the teacher in preparing a new lesson - send a message by keywords and phrases in the next paragraph.
Questions and tasks
This lesson is devoted to the generalization and systematization of knowledge on the topic “Classes inorganic substances" The teacher will tell you how you can get a substance of another class from substances of one class. The acquired knowledge and skills will be useful for drawing up reaction equations along chains of transformations.
During chemical reactions a chemical element does not disappear, atoms move from one substance to another. Atoms chemical element as if transferred from a simple substance to a more complex one, and vice versa. Thus, so-called genetic series arise, starting with a simple substance - a metal or non-metal - and ending with a salt.
Let me remind you that salts contain metals and acidic residues. So, the genetic series of a metal may look like this:
From a metal, as a result of the reaction of a compound with oxygen, a basic oxide can be obtained, a basic oxide, when interacting with water, gives a base (only if this base is an alkali), and a salt can be obtained from a base as a result of an exchange reaction with an acid, salt or acidic oxide.
Please note that this genetic series is only suitable for metals whose hydroxides are alkalis.
Let us write down the reaction equations corresponding to the transformations of lithium in its genetic series:
Li → Li 2 O → LiOH → Li 2 SO 4
As you know, metals, when interacting with oxygen, usually form oxides. When oxidized by atmospheric oxygen, lithium forms lithium oxide:
4Li + O 2 = 2Li 2 O
Lithium oxide, interacting with water, forms lithium hydroxide - a water-soluble base (alkali):
Li 2 O + H 2 O = 2LiOH
Lithium sulfate can be obtained from lithium in several ways, for example, as a result of a neutralization reaction with sulfuric acid:
2. Chemical information network ().
1. p. 130-131 No. 2.4 from the Workbook in Chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. 8th grade” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; ed. prof. P.A. Orzhekovsky - M.: AST: Astrel: Profizdat, 2006.
2. p.204 No. 2, 4 from the textbook P.A. Orzhekovsky, L.M. Meshcheryakova, M.M. Shalashova “Chemistry: 8th grade,” 2013
In this article we will talk about genetic series of metals. Individual chemical substances It is customary to divide into 2 groups: simple substances And complex.
This diagram gives a simplified idea of genetic sequence of metals.
On top are a group of metals and hydrogen, the structure of which differs from the structure of atoms of other elements. There is 1 electron in the outer level, like alkali metals, but at the same time there is not enough 1st electron to fill the outer level.
Based on genetic line metals form basic oxides. Hydrogen forms a specific amphoteric oxide - water H2O, which, when interacting with the main oxide, gives a base (alkali). Such reactions proceed, as a rule, without changing the oxidation state. With change, only those reactions occur in which complex substances are formed from simple substances:
2 Cu + O 2 = 2 CuO,
Basic oxides react with nonmetals, acidic oxides, acids, acid salts.
Depending on the acid, metal or non-metal, different salts are formed. For example:
Cu(HE) 2 + H 2 SO 4 = SO 4 .
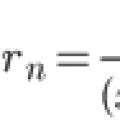 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A