Most of the impurities are found in water. Large electronic collection of recipes for catering establishments
Water is a universal solvent. Its interaction with anything affects the composition of the liquid. Water can contain a huge amount of a wide variety of impurities - about 70 thousand substances. You can purify water from harmful impurities using special devices. This article will tell you about what harmful impurities may be contained in water and how to get rid of them.
From this article you will learn:
How do non-metallic contaminants occur in water?
What metallic contaminants are there in water?
Non-metallic contaminants in water
Among the many non-metallic substances in water can be:
Let's look at each of these chemical elements in more detail.
Fluorine
The chemical element plays an important role in the composition of the human body. It is found in humans as fluoropathite and is a component of our bones and teeth. Fluoropatite helps us absorb iron and protect tooth enamel from caries. However, everything needs balance. Excess fluoride in the body can provoke fluorosis, which leads to the appearance of dark spots on the teeth, changes in the composition of the bones (deforming them, the ligamentous apparatus also undergoes severe changes). Harmful impurities in water, such as fluorine and manganese, are recognized by the yellowish color of the liquid and its astringent taste.
Chlorine
Chlorine enters the liquid through the disinfection system used by water treatment plants. Chlorination helps make water suitable for domestic use. However, drinking such water is not recommended, as this will lead to a decrease in the body’s immune system and can cause an allergic reaction, bronchial asthma, cardiovascular diseases, atherosclerosis and even cancer. Harmful impurities in water, which are derivatives of chlorine, have carcinogenic properties. If the pool water is chlorinated, contact with your teeth can cause enamel erosion and dark stains.
Bromine
This element is quite often found in nature in the composition chemical compounds. It can also be found in our body: in the blood, urine, saliva, even in the brain and liver. Bromine enters water from industrial wastewater. Liquids containing bromine elements are dangerous to drink because they can cause malfunctions nervous system person. In addition, such water can cause bromoderma - skin rashes.
Bor
There are several ways that boron can become part of harmful impurities in water:
from industrial wastewater;
from domestic wastewater;
from natural groundwater.
If you drink water that contains a large number of boron, you can achieve complete dehydration of the body. In addition, this chemical element settles tightly in the human body and is difficult to remove, accumulating along with the consumption of contaminated water. Over time, the process can cause intoxication, which is accompanied by symptoms such as vomiting, stomach upset, lack of appetite, peeling and skin rashes.
Iodine
Iodine can be part of harmful impurities in water:
from wastewater from chemical plants;
from sea fumes;
from igneous rocks.
This chemical element is beneficial to the human body in certain quantities. However, drinking water with a high iodine content is strictly prohibited, as it is dangerous to health. Drinking such water causes weakness and headache, vomiting and rapid heartbeat, and a specific coating appears on the tongue.

Arsenic
Arsenic is very toxic and therefore dangerous to our body. Arsenic harmful impurities in water are extremely undesirable, since drinking such liquid will develop endemic goiter. If you continue to drink water contaminated with arsenic, death is inevitable. This element evaporates during volcanic eruptions and enters the atmosphere. It appears as part of harmful impurities in water due to toxic sediments and by leaching from mineral springs in nature.
This same group of harmful impurities in water includes pesticides, fertilizers, and mechanical particles, which will be discussed below.
- Pesticides.
Many harmful impurities in water can cause poisoning. Pesticides, in addition, cause a lot of allergic diseases and diathesis. Consumption of water containing pesticides in large quantities causes chronic diseases and has a detrimental effect on the development of children, causing them various abnormalities.
- Chemical fertilizers.
Phosphates, nitrates, nitrites and polyphosphates - all these are chemical fertilizers. As a rule, they appear in water during its extraction. If there are not so many harmful impurities in the water, such as chemical fertilizers, then its consumption will cause weakness and drowsiness. However, by continuing to consume this liquid, a person risks serious poisoning, illness and even death.
- Large mechanical impurities.
Large mechanical impurities in water are sand, clay, rust particles, mineral deposits, etc. These impurities harm not only humans, but also various household appliances and plumbing fixtures.
Metal contaminants in water
This section is devoted to studying the issue of harmful metal impurities contained in the water entering our homes through the plumbing. We will talk about iron, manganese, chromium, mercury, lead, calcium and magnesium.
- Iron.
Iron is found in huge quantities in both artesian and surface waters. If drinking water contains too high a percentage of iron, then there is a high likelihood of liver disease, decreased reproductive performance, and an increased risk of heart attack and allergic reactions.
Iron contaminants in water also negatively affect household appliances and water pipes due to the development of bacteria and oxidative processes. Iron can also accumulate in the human body (in internal organs and muscles).
- Manganese.
Manganese is also quite often found as part of harmful impurities in water. It develops anemia and has a detrimental effect on the human nervous system.
- Chromium.
Scientists have proven that a high chromium content in water contributes to rapid development cancer in the human body. This element is extremely dangerous due to its toxicity.
- Mercury.
This chemical element and its compounds negatively affect protein metabolism in the human body, and mercury also disrupts the functioning of the central nervous system. Over time, the kidneys and liver fail, and gastrointestinal diseases occur. The so-called methylmercury is an extremely dangerous harmful impurity in water. It causes Minamata disease, which is accompanied by symptoms such as impaired hearing, motor skills, and paralysis develops over time.
- Lead.
Lead is considered the most toxic metal, which also allows us to classify it as a harmful impurity in water. It is deposited in the bones of the human body, disrupting the functioning of the central nervous system and reducing immune protection. Extremely dangerous for children under 6 years of age.
- Calcium and magnesium.
As you know, high levels of calcium and magnesium make water hard and unsuitable for drinking. Boiling hard water causes scale to form on the walls of the vessel. Delamination of accumulated scale causes malfunctions in the operation of household appliances. American scientists conducted a study, thanks to which it was possible to establish that a layer of scale one and a half millimeters thick reduces heat transfer by 15%, a scale thickness of three millimeters - by 25%, etc. In addition, harmful impurities in water such as calcium and magnesium , require up to 25% more electricity to heat the liquid.
Over 90% of heating devices become faulty if they are in constant contact with hard water. Calcium and magnesium dissolved in water have a detrimental effect on the quality of the laundry washed in it, reducing its “life” by 15–30%, while increasing the amount of washing powder consumed by 30%. If you use special cosmetics while washing, their beneficial properties become ineffective when in contact with hard water.
When washing with hard water for a long time, human skin suffers greatly. This leads to clogging of pores, weakening the protective properties of the fatty film on the surface of the skin, which causes peeling, irritation and the appearance of various rashes. In addition to the skin itself, the hair also suffers, which is accompanied by symptoms such as itching and dandruff. The hair itself becomes dry and hard to the touch, stops “obeying”, and visually takes on an unkempt appearance.
Harmful impurities in water, such as calcium and magnesium, can accumulate in the body, leading over time to the deposition of kidney stones and blockage of blood vessels. Do not drink hard water under any circumstances unless you want serious health problems.
How to purify water from harmful impurities
Advocacy
You can clean water from harmful impurities by letting it sit for 5 hours. The chlorine contained in the water evaporates during this time, while the salts end up at the bottom of the vessel. Only after such a procedure can you safely boil the water by carefully pouring it into the kettle. Boiling will completely evaporate such harmful impurities in water as chlorine, and will also help rid the liquid of unwanted microorganisms. The remaining salts in the water after five hours of infusion will settle on the walls of the kettle in the form of scale.
Buying a small household filter
You can also purify water from harmful impurities using a household filter. The operation of this design is very similar to the principle of industrial liquid filtration. A small household filter for purifying water from harmful impurities is a kind of jug with two levels and a removable module. The first level of the jug contains tap water, which is further purified by passing through a removable module. Purified water is located in the second level of the household filter. According to manufacturers, a small household filter purifies liquid from harmful impurities in water such as sand, clay, rust, pesticides, phenols, chlorine, and also kills bacteria dangerous to human health. The removable module itself must be changed every quarter.
Making your own filter
You can also purify water from harmful impurities using a filter made by yourself. It can probably come in handy when camping or in the country. You will need: activated carbon, ordinary gauze, a plastic bottle, a vessel for purified liquid, scissors or a knife. First, take a plastic bottle and cut off the bottom. Next, prepare several layers of gauze and place them in the neck of the bottle. We wrap the activated carbon with the remaining gauze and place the same in a bottle turned upside down. The neck must be located strictly above the vessel for the purified liquid. Thus, you can purify water from harmful impurities by pouring it from the cut-off bottom of the bottle. The water will be purified by passing through layers of activated carbon and gauze.
Freezing
You can purify water from harmful impurities not only by boiling it, but also by freezing it. To rid water of dangerous microorganisms, simply place it in the freezer for 10 hours. At the end of the freezing time, the container with water thaws at room temperature. The remaining small piece of ice must be thrown away, as it contains harmful impurities in the water. Purified water can be consumed by adding 100 grams of salt per liter. Some sources indicate that melt water helps prolong youth and improve the general condition of the body.
Use of silver
You can also purify water from harmful impurities using silver. It's no secret that this metal has bactericidal properties. In order to purify water from contents harmful substances, it is enough to place a silver object of the highest standard in a vessel with liquid, because a metal with a dubious composition can have the opposite effect on the water. However, even silver of the highest standard must be kept in water for a certain amount of time so that it does not become toxic to health.
Use of silicon
You can purify water from harmful impurities not only with silver, but also with silicon. This non-metal also has bactericidal properties. Before using it to purify water, it must be thoroughly rinsed and only then placed in a container with water, covering the surface of the container with gauze. The cleaning procedure will be carried out over several days. At this time, it is important to keep the water container away from direct contact. sun rays. Under no circumstances should water purified with silicon be boiled! The container with purified water should be stored closed so that the liquid remains fresh for as long as possible.
Purchase of a comprehensive water purification system
Tap water has already gone through pre-treatment stages before entering our homes. In this regard, filters used at home are more likely to belong to a comprehensive water purification system. These designs rid the water of various harmful impurities and microorganisms that remain in the water even after purification at the city station. Some harmful substances get into tap water on the way to our apartments (for example, rust of water pipes).
Filters for water purification have the following functions:
Purification of water from mechanical impurities (rust, scale, turbidity, sand, dirt, etc.).
Water softening, i.e. reducing the level of salts contained: calcium, magnesium, as well as mercury, lead and many other heavy metals.
The process of eliminating odors and chlorine, getting rid of liquids organic matter, improving taste, eliminating traces of chlorine, petroleum products, phenol, radionuclides, etc.
Water treatment kit from our catalog
As a rule, household water purification filters do not have disinfecting properties, since this process must take place at city waterworks through chlorination.
Thus, you can purify water from harmful impurities using any of the above methods, making the water not only clean, but also healthy. Clean water is the key to good health and well-being.
The Russian market has a lot of companies that develop and implement water treatment systems. Choosing an individual water treatment system is quite difficult if you do not seek help from professionals. You should not try to install the selected water purification system yourself if this is your first time encountering this. After reading a couple of articles on the Internet, you will not become an expert in this field.
It is best to seek help from specialized companies that develop and install water treatment systems. As a rule, such companies offer several types of services: professional advice from a specialist, analysis of water from a well or well, assistance in selecting a water treatment system taking into account individual characteristics, as well as delivery and installation of equipment. In addition to the above services, such companies provide service for water purification filters.
Biokit offers a wide range of reverse osmosis systems, water filters and other equipment that can return tap water to its natural characteristics.
Our company’s specialists are ready to help you:
connect the filtration system yourself;
understand the process of choosing water filters;
select replacement materials;
troubleshoot or solve problems with the involvement of specialist installers;
find answers to your questions over the phone.
Trust water purification systems from Biokit - let your family be healthy!
Introduction
Industrial water treatment is a set of operations that ensure water purification - the removal of harmful impurities from it that are in a dissolved, colloidal and suspended state.
The harmfulness of impurities contained in water is determined by the technological process using water. Water impurities vary in chemical composition and dispersity. Coarse suspensions clog pipelines and equipment, forming traffic jams that can cause accidents. Impurities found in water in a colloidal state clog the membranes of electrolyzers, causing foaming of the water and overflow in the devices. Huge harm to the production cycle
apply salts and gases dissolved in water that form scale
and causing surface destruction of metals due to corrosion.
Thus, industrial water treatment is a complex and lengthy process that includes the following main operations: sedimentation, coagulation, filtration, softening, desalting, disinfection and degassing.
Characteristics of natural waters and their impurities
Water is one of the most common elements on Earth compounds. The total mass of water on the Earth's surface is estimated at 1.39. 10 18 tons. Most of it is found in the seas and oceans. The fresh water available for use in rivers, canals and reservoirs is 2. 10 14 tons. Stationary reserves of fresh water suitable for use account for only 0.3% of the volume of the hydrosphere.
The chemical industry is the largest consumer of water. Modern chemical enterprises consume up to 1 million m 3 of water per day. Water consumption coefficients in (m³/t) in production: nitric acid– up to 200, ammonia – 1500, viscose silk – 2500.
Process water used in production is divided into cooling, process and energy.
Cooling water serves to cool substances in heat exchangers. It does not come into contact with material flows.
Process water in turn, it is divided into medium-forming, leaching and reaction. Media-forming water is used for dissolution, formation of suspensions, movement of products and waste (hydrotransport); rinsing water – for washing equipment, gaseous (absorption), liquid (extraction) and solid products; reaction water - as a reagent, as well as an agent for azeotropic distillation. Process water is in direct contact with material flows.
Energy water used in the production of steam (to power steam generators) and as a working fluid when transferring heat from a source to a consumer (hot water).
Approximately 75% of the water used in chemical industry, is spent on cooling technological equipment. The rest of the water is used mainly as a chemical reagent, extractant, absorbent, solvent, reaction medium, transport agent, feed water in recovery boilers, for the formation of slurries and suspensions, for washing products and equipment.
The main source that satisfies technical and domestic water needs is natural water.
Natural waters are a complex dynamic system containing gases, minerals and organic substances that are in a truly dissolved, colloidal or suspended state.
by chemical composition into organic (humic acids, fulvic acids, lignin, bacteria, etc.) and inorganic (mineral salts, gases N, O, CO, HS, CH, NH, etc.).
by dispersion. There are four groups.
To the first group include suspensions of insoluble substances in water. The size of these impurities ranges from fine suspensions to large particles, i.e. 10 -5 ÷10 -4 cm or more (sand, clay, some bacteria).
To the second group These include colloidal systems, high-molecular substances with particle sizes of 10 -5 ÷10 -6 cm.
To the third group These include molecular solutions in water of gases and organic substances with a particle size of 10 -6 ÷10 -7 cm. These substances are found in water in the form of undissociated molecules.
To the fourth group These include ionic solutions of substances that dissociate into ions in water and have a particle size of less than 10 -7 cm. In a truly dissolved state, there are mainly mineral salts that enrich the water with Na, K, NH, Ca, Mg, Fe, Mn cations and HCO anions, CI, SO, HSiO, F, NO, CO, etc.
The composition and amount of impurities depends mainly on the origin of the water. By origin, atmospheric, surface and underground waters are distinguished.
Atmospheric waters– rain and snow waters are characterized by a relatively low content of impurities. These waters contain mainly dissolved gases (N, CO, O, industrial emissions gases) and are almost completely free of dissolved salts. Atmospheric water is used as a source of water supply in waterless and arid areas.
Surface water– these are the waters of open reservoirs: rivers, lakes, seas, canals, reservoirs. The composition of these waters includes soluble gases, minerals and organic substances, depending on climatic, soil and geological conditions, agricultural practices, industrial development and other factors.
Sea water has a high salinity content and contains almost all the elements found in earth's crust. Most of all sea water contains sodium chloride (up to 2.6% of all salts).
The groundwater– waters of artesian wells, wells, springs, geysers – are characterized by a significant content of mineral salts leached from soil and sedimentary rocks, and a small amount of organic substances. The filtering capacity of soils determines the high transparency of groundwater.
Depending on the salt content, natural waters are divided into fresh water– salt content up to 1 g/kg; brackish – 1 ÷ 10 g/kg and salty – more than 10 g/kg.
Waters are also distinguished by the predominant anion in them: hydrocarbonate type of waters with the predominant anion HCO or the sum of the anions HCO and CO; sulphate waters; chloride waters. The rivers of the central zone of the European part of Russia are mainly of the hydrocarbonate type.
1 | | | | | | | | | |
Introduction
Water preparation is a responsible task, since the reliability and cost-effectiveness of equipment operation depends on its quality. In order for the equipment of the boiler-turbine shop to operate for a long time without deposits in the boiler tubes and the flow part of the turbine, the concentrations of individual components in the feed water must be in the range from 5 to 100 μg/kg. It is possible to obtain such water in the required quantities only by using advanced methods of processing it.
The highest demands are placed on the quality of water used to fill the circuit of a steam turbine plant and replenish it during operation.
Classification of impurities contained in source water
Water can be divided into:
Atmospheric (rain, fog, snow);
Surface (rivers, lakes, ponds, swamps);
Underground (artesian wells, mine wells);
Salt water (sea, ocean).
Atmospheric water falling onto the earth's surface in the form of precipitation contains gases absorbed from the air, oxygen, nitrogen, carbon dioxide, organic and inorganic substances. In industrial areas and large populated areas atmospheric precipitation contains sulfur oxides, dust particles and soot. The total salt content of atmospheric precipitation is 10-50 mg/kg. Getting to the surface of the earth and seeping through the ground, atmospheric water meets mineral salts (CaCO3, NaCl, Na2SO4, MgSO4), gases and organic substances. Some organic substances, with the help of bacteria, react with oxygen dissolved in water, forming mineral acids (sulfuric, nitric, etc.). The easiest to dissolve in groundwater are NaCl, Na2SO4, MgSO4. Hardly soluble calcium, magnesium and iron carbonates in the presence of free carbon dioxide form bicarbonates, dissociating into Ca2+, Mg2+, Fe2+ cations and HCO3 anions. The most common in natural waters are calcium and magnesium bicarbonates. Groundwater is highly diverse chemical composition. The degree of their mineralization ranges from 100/mg/kg to several grams per 1 kg. The most mineralized waters are the oceans and open seas. Their salt content is approximately 7.5-35 g/kg. In summer and winter, watercourses are fed mainly by underground runoff and, therefore, the concentration of dissolved salts increases at this time of year. Spring flood waters are characterized by a very low salt content of water, but at the same time the concentration of coarse and colloidal impurities increases sharply due to their washing from the surface by melt water.
The variety of impurities in natural waters does not allow them to be classified according to any single criterion, therefore it is customary to classify these impurities according to several criteria. The number of these classifications gives a fairly objective description of them.
By degree of dispersion:
Coarsely dispersed (particle sizes - more than 100 nm),
Colloidal dispersed (have small sizes from 1 to 100 nm),
Truly dissolved (presented in the form of individual ions or molecules or complexes consisting of several molecules).
Coarse water impurities(sand, clay, etc.), also called suspensions or suspended substances, remaining suspended for a long time, causing turbidity in the water. In natural waters, coarse impurities are present in the form of sand, silt, plankton, in process waters - in the form of corrosion products, sludge, and oil products. The larger the particle size of coarse impurities, the faster sedimentation equilibrium is established and the easier they are separated from the water during settling and filtration. Colloidal particles are not released from water under the influence of gravity and are not retained by conventional filter materials.
In natural waters in colloidal dispersed state There are various derivatives of silicic acid and iron, organic humic substances washed out of the soil.
To ion-disperse or molecular-disperse substances include salts, acids, alkalis dissolved in water in the form of ions, Ca2+, Mg2+, N +, K +, 2 SO4, Cl-, NO3, NO2, HCO3, HSiO 3, and molecules of dissolved gases O2, CO2, N2, etc. d. The ionic composition of impurities is characterized by the presence of corresponding cations and anions. Sodium and potassium ions with anions of natural waters do not form sparingly soluble simple salts and practically do not undergo hydrolysis, therefore they are classified as stable impurities. Calcium and magnesium ions form poorly soluble compounds with some anions found in water. When natural water is used and the associated change in the initial concentrations of cations and anions, for example, during evaporation or a decrease in solubility with increasing temperature, poorly soluble calcium and magnesium salts are released on heat transfer surfaces in the form of a solid phase. In technological processes of water preparation, to reduce the concentration of calcium and magnesium, the formation of their sparingly soluble compounds is often used, which are removed from the water before it enters the water-steam path. Iron ions are characterized by polyvalency and can be found in various forms. In deep waters, iron ions are found mainly in the form of Fe2+, which do not form difficultly soluble salts with most ions. In waters of surface sources, where the concentration of dissolved oxygen is much higher, Fe2+ ions are oxidized to Fe3+ ions, which during hydrolysis form poorly soluble Fe(OH)3. In surface waters, iron can also be part of organic complex compounds. The presence of iron compounds in water in elevated concentrations creates conditions for the development of iron bacteria that form bumpy colonies on the walls of pipelines. The concentration of iron in source water may increase during its transportation through steel and cast iron pipes due to contamination with corrosion products. Anions carbonic acid HCO3 and 2 CO3 are the most important components of the salt components of water, since they determine the behavior of various impurities in it. In addition to HCO3 and 2 CO3, natural waters also contain “free” carbon dioxide, which is in the form of CO2 gas dissolved in water and its hydrate - H2CO3 molecules.
According to their chemical nature, impurities are divided into:
Gas (gases N2, O2, CO2, CH4, H2S),
Mineral (dissolved mineral salts)
Organic (humic substances, tannins, proteins, fats, essential oils). Natural waters are divided into:
Fresh (up to 1g/kg),
Salty (1-10 g/kg)
Salty (more than 10 g/kg).
Fresh waters can be divided into:
Low-mineralized (less than 0.2 g/kg),
Medium mineralization (0.2-0.5g/kg)
Increased mineralization (0.5-1g/kg).
By total hardness value natural waters are classified as follows:
F less than 1.5 - water with low hardness,
F = 1.5 - 3 with medium hardness,
F = 3 - 6 - water with increased hardness,
F = 6 - 12 - water with high hardness,
w more than 12 - water with very high hardness.
All natural waters are divided into three classes according to the nature of the predominant anion in the water :
- hydrocarbonate (HCO3 anion),
- sulfate (SO4),
Chloride (chlorine ion).
Over 80% of all rivers in Russia are characterized by waters of the hydrocarbonate class. According to the degree of contamination with organic substances, natural waters can be divided into four groups: less than 5 - low, 5-10 - medium, 10-20 high, over 20 - strong.
04.09.2014 00:40
Main water problems.
Increased turbidity.
Increased turbidity is typical for both artesian, well, and tap water. Turbidity in water is caused by suspended and colloidal particles that scatter light. These can be either organic or inorganic substances or both at the same time. In most cases, suspended particles themselves do not pose a serious threat to health, but for modern equipment they can cause premature failure. Increased turbidity of tap water is often associated with mechanical separation of pipeline corrosion products and biofilms developing in the central water supply system. The reason for the increased turbidity of artesian waters is usually clay or limestone suspensions, as well as insoluble oxides of iron and other metals formed upon contact with air.
The quality of water from wells is the least stable, since groundwater is affected external factors. High turbidity of water from wells may be associated with the entry into groundwater of sparingly soluble natural organic substances from soils with technogenic pollution. High turbidity negatively affects the effectiveness of water disinfection, as a result of which microorganisms attached to the surface of particles survive and continue to develop on their way to the consumer. Therefore, reducing turbidity often improves the microbiological quality of water.
Iron in water.
High iron content in tap water is associated with various reasons. These impurities get into tap water as a result of corrosion of pipelines or the use of iron-containing coagulants at water treatment stations, and into artesian waters as a result of contact with iron-containing minerals. The iron content in artesian waters on average exceeds the standard value by 2-10 times. In some cases, the excess can be up to 30-40 times. Usually, immediately after receiving, artesian water does not bear visible signs of the presence of iron compounds, however, upon contact with air oxygen, a yellow color may appear after 2-3 hours, and with longer settling, the formation of a light brown precipitate may be observed. All this is the result of an oxidative process, during which heat is released. Stimulating the development of glandular bacteria in artesian water.
Manganese in water.
Manganese impurities in water from artesian wells are detected simultaneously with iron impurities. The source of their supply is the same - the dissolution of manganese-containing minerals. Exceeding the manganese content in drinking water worsens its taste, and when such water is used for domestic needs, the formation of dark deposits is observed in pipelines and on the surfaces of heating elements. Washing your hands with water high in manganese leads to an unexpected effect - the skin first turns gray and then completely black. With prolonged exposure to water with a high manganese content, the risk of developing diseases of the nervous system increases.
Oxidability and color.
Increased oxidation and color of surface and artesian water supply sources indicate the presence in the water of impurities of natural organic substances - humic and fulvic acids, which are products of the decomposition of living and inanimate nature. High levels of organic matter in surface waters are recorded during the period of algae decay (July - August). One of the concentration characteristics organic pollution is permanganate oxidation. In the area of peat occurrence, especially in the regions of the far north and eastern Siberia, this parameter can exceed the permissible values by tens of times. Natural organic substances themselves do not pose a health threat. However, with the simultaneous presence of iron and manganese in water, their organic complexes are formed, making it difficult to filter them by aeration, that is, oxidation with atmospheric oxygen. Presence of organic substances in water natural origin makes it difficult to disinfect water using oxidative methods, as disinfection by-products are formed. These include trihalomethanes, haloacetic acid, haloketones and haloacetonitrile. Most studies show that substances in this group have a carcinogenic effect and also have Negative influence on the organs of the digestive and endocrine systems. The main way to prevent the formation of by-products of water disinfection is its deep purification from natural organic substances before the chlorination stage, but traditional methods of centralized water treatment do not provide this.
The smell of water.
The smell of tap, artesian and well water makes it unsuitable for consumption. When assessing the quality of water, consumers focus on individual sensations of smell, color and taste.
Drinking water should not have any odor noticeable to the consumer.
The cause of the smell of tap water is most often dissolved chlorine entering the water at the disinfection stage during centralized water treatment.
The smell of artesian water may be associated with the presence of dissolved gases - hydrogen sulfide, sulfur oxide, methane, ammonia and others.
Some gases may be products of the vital activity of microorganisms or the result of technogenic pollution of water supplies.
Well water is most susceptible to foreign contaminants, so often an unpleasant odor can be associated with the presence of petroleum products and traces of household chemicals in the water.
Nitrates
Nitrates in well and artesian water can pose a serious threat to consumer health, since their content can be several times higher than the current drinking water standard.
The main reason for the entry of nitrates into surface and groundwater is the migration of fertilizer components in soils.
Drinking water with a high content of nitrates leads to the development of methemoglobinemia, a condition characterized by the appearance in the blood of an increased level of methemoglobin (>1%), impairing the transfer of oxygen from the lungs to the tissues. As a result of nitrate poisoning, the respiratory function of the blood is sharply impaired and the development of cyanosis may begin - a bluish coloration of the skin and mucous membranes.
In addition, a number of studies have shown the negative effect of nitrates on the absorption of iodine in the body and the carcinogenic effect of the products of their interaction with various substances in the human body.
Hardness of water.
Water hardness is mainly determined by the concentration of calcium and magnesium ions in it.
There is an opinion that hard water does not pose a risk to the health of consumers, but this contradicts the conclusions of many years of research by one of the leading experts on nutrition, American researcher Paul Breg. He believes that he was able to establish the cause of early aging of the human body. The reason for this is hard water. According to Paul Breguet, hardness salts “slag” blood vessels in the same way as pipes through which water with a high content of hardness salts flows. This leads to a decrease in the elasticity of blood vessels, making them fragile. This is especially evident in the thin blood vessels of the cerebral cortex, which, according to Breg, leads to senile insanity in older people.
Hard water creates whole line household problems, causing the formation of sediments and deposits on the surface of pipelines and working elements of household appliances. This problem is especially relevant for appliances with heating elements - hot water boilers (boilers), washing machines and dishwashers.
When using hard water in everyday life, the layer of deposits of calcium and magnesium salts on heat transfer surfaces constantly grows, as a result of which the efficiency of heat transfer decreases and the consumption of thermal energy for heating increases. In some cases, overheating of working elements and their destruction is possible.
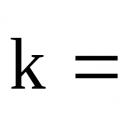 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe