Yakimova chemistry laboratory workshop. Workshop on general chemistry
Federal Agency for Education Tomsk State University of Architecture and Civil Engineering
I.A. KURZINA, T.S. SHEPELENKO, G.V. LYAMINA, I.A. BOZHKO, E.A. VAYTULEVICH
LABORATORY PRACTICUM ON GENERAL AND INORGANIC CHEMISTRY
Tutorial
Publishing house of Tomsk State University of Architecture and Civil Engineering
UDC 546 (076.5) L 12
Laboratory workshop on general and inorganic chemistry [Text]: textbook / I.A. Kurzina, T.S. Shepelenko, G.V. Lyamina [and others]; under. ed. I.A. Kurzina.
Tomsk: Publishing house Tom. state architect-builds University, 2006. – 101 p. – ISBN 5–93057–172–4
IN The textbook provides theoretical information on the main sections of the general course
And inorganic chemistry (classes of inorganic compounds, basic laws and concepts of chemistry, energy effects of chemical reactions, chemical kinetics, solutions, electrochemistry, basic properties of some elements of groups I – VII of D.I. Mendeleev’s periodic table). The experimental part describes the methods for performing seventeen laboratory works. The manual will allow students to more effectively prepare for practical classes and save time when preparing reports on laboratory work. The textbook is intended for all specialties of all forms of education.
Ill. 14, table. 49, bibliogr. 9 titles Published by decision of the editorial and publishing council of TSASU.
Reviewers:
Associate Professor of the Department of Analytical Chemistry of the Chemical Faculty of TSU, Ph.D. V.V. Shelkovnikov Associate Professor, Department of General Chemistry, TPU, Ph.D. G.A. Voronova Associate Professor, Department of Chemistry, TSASU, Ph.D. T.M. Yuzhakova
University, 2006
Introduction........................... |
||||
Rules for working in a chemical laboratory.................................................................... ................... |
||||
Laboratory work No. 1. Classes of inorganic compounds................................... |
||||
Laboratory work No. 2. Determination of molecular mass of oxygen................... |
||||
Laboratory work No. 3. Determination of the thermal effect of a chemical reaction..... |
||||
Laboratory work No. 4. Kinetics of chemical reactions............................................ |
||||
Laboratory work No. 5. Determination of solution concentration. Hardness of water... |
||||
Laboratory work No. 6. Reactions in electrolyte solutions. Hydrolysis of salts......... |
||||
Laboratory work No. 7. Electrochemical processes............................................. |
||||
Laboratory work No. 8. Chemical properties of metals. Corrosion........................ |
||||
Laboratory work No. 9. Aluminum and its properties.................................................... |
||||
Laboratory work No. 10. Silicon. Hydraulic binders................................. |
||||
Laboratory work No. 11. Nitrogen and phosphorus compounds............................................. |
||||
Laboratory work No. 12. Sulfur and its properties............................................................... |
||||
Laboratory work No. 13. Chromium subgroup elements.............................................. |
||||
Laboratory work No. 14. Halogens ................................................. ........................................ |
||||
Laboratory work No. 15. Manganese subgroup elements......................................... |
||||
Laboratory work No. 16. Iron family subgroup............................................. |
||||
Conclusion................................................. ........................................................ ........................ |
||||
Annex 1. List of essential acids........................................................................ |
||||
Appendix 2. Characteristics acid-base indicators ................................... |
||||
Appendix 3. The most important physico-chemical quantities ..................................................... |
||||
Appendix 4. The most important physico-chemical constants ..................................................... |
||||
Appendix 5. Relationship between units of measurement........................................... |
||||
Appendix 6. Prefixes of multiples and submultiples.................................................... |
||||
Appendix 7. Cryoscopic and ebullioscopic constants of some races |
||||
creators ..................................................... ........................................................ ........................... |
||||
Appendix 8. |
electrolytic dissociation (α) of the most important |
|||
electrolytes in 0.1 N solutions at 25 °C............................................................................. |
||||
Appendix 9. |
Constants |
dissociation |
some electrolytes in water |
|
solutions at 25 °C............................................................................................................... |
||||
Appendix 10. |
solubility |
inorganic compounds at |
||
room temperature......................................................................................................... |
||||
Appendix 11. Electrochemical voltage range and standard electrode |
||||
potentials at 25 °C........................................................................................................... |
||||
Appendix 12. Processes occurring during the electrolysis of aqueous solutions |
||||
salts ..................................................... ........................................................ .................................... |
||||
Appendix 13. Periodic table of elements D.I. Mendeleev ..................................... |
||||
INTRODUCTION
Chemistry refers to the natural sciences that study the material world around us. The material objects that make up the subject of the study of chemistry are chemical elements and their various compounds. All objects of the material world are in continuous movement (change). There are various forms of motion of matter, including the chemical form of motion, which is also the subject of the study of chemistry. The chemical form of movement of matter includes various chemical reactions (transformations of substances). So, chemistry is the science of the properties of chemical elements and their compounds and the laws of transformation of substances.
The most important applied aspect of modern chemistry is the targeted synthesis of compounds with the necessary and previously predicted properties for their subsequent use in various fields of science and technology, in particular for the production of unique materials. It should be noted that chemistry as a science has come a short way to the present day - approximately starting from the 60s of the 19th century. Over a period that lasted one and a half centuries, a periodic classification of chemical elements and the doctrine of periodicity were developed, a theory of the structure of the atom, a theory of chemical bonding and the structure of chemical compounds was created, such important disciplines for describing chemical processes as chemical thermodynamics and chemical kinetics appeared, quantum chemistry arose, radiochemistry, nuclear physics. Chemical research has expanded so that individual branches of chemistry - inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, biochemistry, agrochemistry etc. – have become self-
worthwhile independent sciences.
This educational and methodological manual includes two main sections of modern chemistry: “General Chemistry” and “Inorganic Chemistry”. General chemistry lays the theoretical foundations for understanding the diverse and complex picture of chemical phenomena. Inorganic chemistry introduces into the concrete world of substances formed by chemical elements. The authors sought to cover the main issues of the general chemistry course in as brief a form as possible. Considerable attention is paid to theoretical sections of general chemistry: basic laws and concepts of chemistry, chemical thermodynamics, chemical kinetics, properties of solutions, electrochemistry. The section “Inorganic Chemistry” examines the basic properties of elements of groups I–VII of the periodic table by D.I. Mendeleev. The appendices give the basic physical and chemical properties of inorganic substances. This teaching aid is designed to help students master the basic principles of chemistry, acquire skills in solving typical problems and conducting experiments in a chemical laboratory.
When conducting laboratory work, it is very important to observe safety precautions. Work with this teaching aid should begin with familiarization with the basic rules of work in a chemical laboratory.
RULES OF WORK IN THE CHEMICAL LABORATORY
Safety requirements before starting work:
1. Before performing laboratory work, it is necessary to familiarize yourself with the physical and technical properties of the substances used and formed during the chemical reaction, as well as with the instructions and rules for handling them.
2. Keep the work area clean and tidy. Only the necessary equipment and a workbook should be on the desktop.
Safety requirements during operation:
1. You should start performing the experiment only when its purpose and objectives are clearly understood, when the individual stages of the experiment have been thought through.
2. Work with toxic, volatile and caustic substances must be carried out only in a fume hood.
3. During all work, exercise maximum caution, remembering that carelessness
And inattention may result in an accident.
4. Do not lean over a vessel with boiling liquid. The heated test tube must be held with the opening away from you, as liquid may escape. Warm the contents throughout the test tube, not just from the bottom.
5. After using a reagent, it must be immediately put back in place so as not to create chaos in the workplace and not to mix up the reagents when arranging them at the end of classes.
6. When diluting concentrated sulfuric acid, it is necessary to pour the acid in small portions into water, and not vice versa.
7. It is prohibited to work with flammable substances near switched on electrical appliances and burning alcohol lamps or burners.
8. You should sniff the substance by directing the vapor towards you with a movement of your hand, rather than inhaling it deeply.
9. You cannot use substances for experiments from cans, packages and droppers without labels or with illegible inscriptions.
10. If acid or alkali comes into contact with the skin, it is necessary to rinse the burned area with plenty of water, and then - in case of acid burns - 3% soda solution, and for burns with alkalis - 1% boric acid solution.
11. If the reagent gets into your eyes, rinse them with a stream of water, and in case of gas poisoning, provide the victim with a flow of fresh air.
12. To avoid poisoning, it is strictly forbidden to store or eat food or smoke in the work rooms of chemical laboratories.
Safety requirements after completion of work:
It is necessary to remove everything spilled, broken and scattered from the table and floor. After completing the experiment, the workplace must be put in order. Do not throw granules and pieces of metal into the sink, but put them in a special container and hand them over to the laboratory assistant. No substances from the laboratory should be taken home. After finishing work, you must
Wash your hands thoroughly. Report all violations of safety rules and unforeseen situations to the teacher immediately!
I have read and agree to comply with the safety rules. Student signature:
Conducted instructions, checked knowledge of safety rules. Signature of the teacher:

Laboratory work No. 1
CLASSES OF INORGANIC COMPOUNDS
Purpose of the work: to study classes of inorganic compounds, methods of their preparation and chemical properties.
Theoretical part
All chemicals are divided into two groups: simple and complex. Simple substances consist of atoms of one element (Cl2, O2, C, etc.). Complex compounds include two or more elements (K2 SO4, NaOH, HNO3, etc.). The most important classes of inorganic compounds are oxides, hydroxides and salts (figure).
Oxides are compounds consisting of two elements, one of which is oxygen. Based on their functional characteristics, oxides are divided into salt-forming and non-salt-forming (indifferent). Non-salt-forming are called oxides that do not form hydrate compounds and salts (CO, NO, N2 O). Salt-forming oxides According to their chemical properties, they are divided into basic, acidic and amphoteric (figure). The chemical properties of the oxides are presented in table. 1.
Na2O; MgO; CuO.
Acidic oxides form all non-metals (except F) and metals with a high oxidation state (+5, +6, +7), for example SO3; P2 O5 ; Mn2 O7; CrO3.
Amphoteric oxides form some metals in the oxidation state +2 (Be, Zn, Sn, Pb) and almost all metals in the oxidation state +3 and +4 (Al, Ga, Sc, Ge, Sn, Pb, Cr, Mn).
Table 1
Chemical properties of oxides
Basic oxides |
Acidic oxides |
||
Basic oxide + H2 O → Base |
Acidic oxide + H2 O → Acid |
||
CaO+H2O → Ca(OH)2 |
SO3 +H2 O → H2 SO4 |
||
Basic oxide + acid. oxide → Salt |
Sour. oxide + Basic oxide → Salt |
||
CaO+CO2 → CaCO3 |
SO3 + Na2 O → Na2 SO4 |
||
Basic oxide + acid → salt + H2 O |
Sour. oxide + base → salt + H2 O |
||
CaO+H2 SO4 → CaSO4 +H2 O |
SO3 + 2NaOH → Na2 SO4 +H2 O |
Amphoteric oxides
1. Amphoteric oxide + H 2 O →
2. Amph. oxide + acid. oxide → Salt 2. Amph. oxide + Basic oxide → Salt
ZnO + N2 O5 → Zn(NO3 )2 |
ZnO2 + Na2 O → Na2 ZnO2 (in melt) |
3. Amph. oxide + Acid → Salt + H2 O 3. Amph. oxide + base → salt + H2 O |
|
ZnO + H2 SO4 → ZnSO4 +H2 O |
ZnO+2NaOH → Na2 ZnO2 +H2 O (in melt) |
ZnO+2NaOH 2 → Na2 (in solution) |
|

INORGANIC COMPOUNDS |
||||
Basic |
||||
IA: Li, Na, K, Rb, Cs |
Me2 O (Me=Li, Na, K, Rb, Cs) |
|||
IIA: Mg, Ca, Sr, Ba |
MeO (Me=Mg, Ca, Sr, Ba, Cu, Ni) |
|||
AMPHOTERIC |
Salt-forming |
Amphoteric |
||
EO (E=Be, Zn, Sn, Pb) |
||||
E2 O3 (E=Al, Ga, Cr) |
||||
EO2 (E=Ge, Pb) |
||||
Acidic |
||||
Cl2O |
||||
EO2 (E=S, Se, C, Si) |
||||
NOBLE |
E2 O3 (E=N, As) |
|||
E2 O5 (E=N, P, As, I) |
||||
EO3 (E = S, Se) |
||||
VIIIA: He, Ne, Ar |
||||
Non-salt-forming |
||||
CO, NO, N2O, SiO, S2O |
||||
NON-METALS |
||||
Basic (grounds) |
||||
VA: N2, P, As |
||||
VIA: O2, S, Se |
MeOH (Me=Li, Na, K, Rb, Cs) |
|||
VIIA: F2, Cl2, Br2, I2 |
Me(OH)2 (Me=Mg, Ca, Sr, Ba, Cu, Ni) |
|||
Amphoteric |
||||
E(OH)2 (E=Be, Zn, Sn, Pb) |
||||
E(OH)3 (E=Al, Cr) |
||||
HYDROXIDES |
Acidic (acids) |
|||
Oxygen- |
Acid-free |
|||
HEO2 (E=N, As) |
(E=F, Cl, Br, I) |
|||
H3 AsO3 |
||||
H2 EO3 (E=Se, C) |
||||
HEO3 (E=N, P, I) |
||||
H3 EO4 (E=P, As) |
||||
H2 EO4 (E=S, Se, Cr) |
||||
HEO4 (E=Cl, Mn) |
||||
Basic salts (hydroxy salts) |
||||
FeOH(NO3 )2 , (CaOH)2 SO4 |
||||
Medium salts (normal) |
||||
Na2 CO3, Mg(NO3)2, Ca3 (PO4)2 |
||||
Acid salts (hydrosalts) |
||||
NaHSO4, KHSO4, CaH2 (PO4)2 |
||||
Classification of inorganic compounds |
||||

Hydroxides are chemical compounds of oxides with water. Based on their chemical properties, basic hydroxides, acidic hydroxides and amphoteric hydroxides are distinguished (see figure). The main chemical properties of hydroxides are given in table. 2.
Basic hydroxides or bases are substances that, upon electrolytic dissociation in aqueous solutions, form negatively charged hydroxide ions (OH–) and do not form other negative ions. Alkali metal hydroxides that are highly soluble in water, except LiOH, are called alkalis. The names of basic hydroxides are formed from the word “hydroxide” and the name of the element in the genitive case, after which, if necessary, the degree of oxidation of the element is indicated in Roman numerals in brackets. For example, Fe(OH)2 is iron (II) hydroxide.
Acidic hydroxides or acids are substances that, when dissociated in aqueous solutions, form positively charged hydrogen ions (H+) and do not form other positive ions. The names of acid hydroxides (acids) are formed according to the rules established for acids (see Appendix 1)
Amphoteric hydroxides or ampholytes are formed by elements with amphoteric properties. Amphoteric hydroxides are called similarly to basic hydroxides, for example, Al(OH)3 - aluminum hydroxide. Ampholytes exhibit both acidic and basic properties (Table 2).
table 2
Chemical properties of hydroxides
Grounds |
|||
to C |
|||
Base → Basic oxide + H2O |
|||
to C |
|||
Ba(OH)2 → BaO + H2O |
|||
Base + Acid. oxide → Salt + H2O |
2. Acid + Basic. oxide →Salt+ H2 O |
||
Ba(OH)2 + CO2 → BaCO3 + H2O |
H2 SO4 + Na2 O → Na2 SO4 + H2 O |
||
3. Base + Acid → Salt + H 2 O
Ba(OH)2 + H2 SO4 → BaSO4 + 2H2 O
Amphoteric hydroxides
1. Amph. hydroxide+acid. oxide→Salt+H2 O 1. Amph. hydroxide+basic oxide → Salt+H2 O
Salts are substances whose molecules consist of metal cations and an acid residue. They can be considered as products of partial or complete replacement of hydrogen in an acid with a metal or hydroxide groups in the base with acidic residues.
There are medium, acidic and basic salts (see figure). Medium or normal salts are products of complete replacement of hydrogen atoms in acids with a metal or hydroxide groups in bases with an acid residue. Acid salts are products of incomplete replacement of hydrogen atoms in acid molecules with metal ions. Basic salts are products of incomplete replacement of hydroxide groups in bases with acidic residues.

The names of medium salts are made up of the name of the acid anion in the nominative case (Adj. 1) and the name of the cation in the genitive case, for example CuSO4 - copper sulfate. The name of acidic salts is formed in the same way as the middle ones, but the prefix hydro- is added, indicating the presence of unsubstituted hydrogen atoms, the number of which is indicated by Greek numerals, for example, Ba(H2PO4)2 - barium dihydrogen phosphate. The names of the main salts are also formed similarly to the names of the middle salts, but the prefix hydroxo- is added, indicating the presence of unsubstituted hydroxo groups, for example, Al(OH)2 NO3 - aluminum dihydroxonitrate.
Work order
Experiment 1. Establishing the nature of the oxides
Experiment 1.1. Interaction of calcium oxide with water (A), hydrochloric acid (B), and sodium hydroxide (C). Check the medium of the resulting solution in experiment (A) using an indicator
(Appendix 2).
Observations: A.
Reaction equations:
Experiment 1.2. Interaction of boron oxide with water (A), hydrochloric acid (B), and sodium hydroxide (C). Experiment (A) is carried out with heating. Check the medium of the resulting solution in experiment (A) using an indicator (Appendix 2).
Observations: A.
Reaction equations:
Experience 2. Preparation and properties of aluminum hydroxide
Experiment 2.1. Interaction of aluminum chloride with sodium hydroxide deficiency
No.
Sections, topics
Number of hours
Work program by class
10 grades
11th grade
Introduction
1. Solutions and methods for their preparation
2. Calculations using chemical equations
3. Determination of the composition of mixtures
4. Determination of the formula of a substance
5. Patterns of chemical reactions
6. Combined tasks
7. Qualitative reactions
Introduction to chemical analysis.
Chemical processes.
Chemistry of elements.
Corrosion of metals.
Food chemistry.
Pharmacology.
Final conference: “The importance of experiment in the natural sciences.”
Total:
Explanatory note
This elective course is intended for students in grades 10 - 11 choosing a natural science direction, designed for 68 hours.
The relevance of the course lies in the fact that its study will allow you to learn how to solve the main types of calculation problems that are provided for in the high school chemistry course and the program of entrance exams to universities, that is, to successfully prepare for the Unified State Exam in chemistry. In addition, the lack of practical training is compensated for. This makes classes exciting and instills skills in working with chemical reagents and equipment, develops observation and the ability to think logically. In this course, an attempt is made to make maximum use of the clarity of a chemical experiment, to enable students not only to see how substances interact, but also to measure in what proportions they enter into reactions and are obtained as a result of the reaction.
Purpose of the course: expanding students' understanding of chemical experiments.
Course objectives:
· Repetition of material covered in chemistry lessons;
· Expanding students’ understanding of the properties of substances;
· Improving practical skills and skills in solving calculation problems of different types;
· Overcoming the formal understanding of some schoolchildren about chemical processes.
During the course, students improve their skills in solving calculation problems, perform qualitative tasks to identify substances found in different bottles without labels, and experimentally carry out chains of transformations.
During the experiment, five types of skills and abilities are formed in the classroom.
1. Organizational skills:
drawing up an experimental plan according to instructions;
determination of the list of reagents and equipment according to the instructions;
preparing a report form according to instructions;
performing an experiment at a given time, using familiar tools, methods and techniques in work;
carrying out self-control according to instructions;
knowledge of the requirements for written documentation of experimental results.
2. Technical skills:
correct handling of known reagents and equipment;
assembly of devices and installations from finished parts according to instructions;
performing chemical operations according to instructions;
compliance with labor safety rules.
3. Measuring skills:
working with measuring instruments in accordance with the instructions;
knowledge and use of measurement methods;
processing of measurement results.
4. Intellectual skills and abilities:
clarifying the purpose and defining the objectives of the experiment;
putting forward an experiment hypothesis;
selection and use of theoretical knowledge;
observation and identification of characteristic signs of phenomena and processes according to instructions;
comparison, analysis, establishment of cause-and-effect relationships,
generalization of the results obtained and - formulation of conclusions.
5. Design skills:
correcting simple problems in equipment, devices and installations under the supervision of a teacher;
use of ready-made equipment, instruments and installations;
production of simple equipment, instruments and installations under the guidance of a teacher;
depiction of equipment, instruments and installations in the form of a picture.
Knowledge control is carried out when solving computational and experimental problems.
The result of the elective course will be the completion of a test work, including the preparation, solution and experimental implementation of a calculation problem or a qualitative task: determining the composition of a substance or the implementation of a chain of transformations.
Introduction (1 hour)
Planning, preparing and conducting a chemical experiment. Safety precautions during laboratory and practical work. Rules for providing first aid for burns and chemical poisoning.
Topic 1. Solutions and methods for their preparation (4 hours)
The importance of solutions in a chemical experiment. The concept of a true solution. Rules for preparing solutions. Technochemical balances and rules for weighing solids.
Mass fraction of solute in solution. Calculation and preparation of a solution with a certain mass fraction of the dissolved substance.
Determination of volumes of solutions using measuring containers and the density of solutions of inorganic substances using a hydrometer. Tables of densities of solutions of acids and alkalis. Calculations of solute mass from known density, volume, and mass fraction of solute.
Changing the concentration of a solute in a solution. Mixing two solutions of the same substance to obtain a solution of a new concentration. Calculation of the concentration of a solution obtained by mixing, the “cross” rule.
Demonstrations. Chemical glassware for preparing solutions (glasses, conical and flat-bottomed flasks, graduated cylinders, volumetric flasks, glass rods, glass funnels, etc.). Preparation of sodium chloride solution and sulfuric acid solution. Technochemical scales, weights. Determining the volume of solutions of acids and alkalis using a graduated cylinder. Hydrometer. Determination of the density of solutions using a hydrometer. Increasing the concentration of sodium hydroxide solution by partially evaporating the water and adding additional alkali to the solution, checking the change in concentration using a hydrometer. Reducing the concentration of sodium hydroxide in a solution by diluting it, checking the change in concentration using a hydrometer.
Practical work. Weighing sodium chloride on a technical chemical balance. Preparation of a solution of sodium chloride with a given mass fraction of salt in the solution. Determining the volume of sodium chloride solution using a graduated cylinder and determining its density using a hydrometer. Determination of the concentration of solutions of acids and alkalis by their densities in the table “Mass fraction of dissolved substance (in%) and density of solutions of acids and bases at 20 °C.” Mixing sodium chloride solutions of various concentrations and calculating the mass fraction of salt, and determining the density of the resulting solution.
Topic 2. Calculations using chemical equations (10 hours)
Practical determination of the mass of one of the reacting substances by weighing or by volume, density and mass fraction of the dissolved substance in the solution. Carrying out a chemical reaction and calculating how to reduce this reaction. Weighing the reaction product and explaining the difference between the practical result obtained and the calculated one.
Practical work. Determination of the mass of magnesium oxide obtained by burning a known mass of magnesium. Determination of the mass of sodium chloride obtained by reacting a solution containing a known mass of sodium hydroxide with an excess of hydrochloric acid.
Practical determination of the mass of one of the reacting substances using weighing, carrying out a chemical reaction and calculation using the chemical equation of this reaction, determining the mass or volume of the reaction product and its yield as a percentage of the theoretically possible.
Practical work. Dissolving zinc in hydrochloric acid and determining the volume of hydrogen. Calcination of potassium permanganate and determination of the volume of oxygen.
Carrying out reactions for substances containing impurities, observing the results of the experiment. Calculations with determination of the mass fraction of impurities in a substance based on the results of a chemical reaction.
Demonstration experiment. Dissolving sodium, calcium in water and observing the results of the experiment to detect impurities in these metals.
Practical work. Dissolving chalk powder contaminated with river sand in a solution of nitric acid.
Determination of the masses of reacting substances, carrying out a chemical reaction between them, studying the reaction products and practical determination of a substance in excess. Solving problems to determine the mass of one of the reaction products from the known masses of the reacting substances, one of which is given in excess.
Demonstration experiment. Combustion of sulfur and phosphorus, determination of the substance that is in excess in these reactions.
Practical work. Carrying out a reaction between solutions of nitric acid and sodium hydroxide containing known masses of reacting substances, determining the excess of the reagent using an indicator.
Topic 3. Determination of the composition of mixtures (2 hours)
Reacting a mixture of two substances with a reagent that reacts with only one component of the mixture. Reacting a mixture of two substances with a reagent that reacts with all components of the mixture. Discussion of the experimental results. Solving problems to determine the composition of mixtures.
Demonstration experiment. Interaction of a mixture of zinc dust and copper filings with hydrochloric acid. Interaction of a mixture of magnesium powder and zinc dust with hydrochloric acid.
Topic 4. Determining the formula of a substance (6 hours)
The concept of the qualitative and quantitative composition of a substance. Calculation of the molecular mass of a substance based on its hydrogen density, etc. and mass fraction of the element. Determining the formula of a substance based on quantitative data of reaction products. Determination of the formula of organic substances based on the general formula of the homologous series.
Topic 5. Patterns of chemical reactions (5 hours)
The concept of thermal processes in chemical reactions. Exo- and endothermic reactions. Calculations using thermochemical equations.
Demonstration. The reaction of diluting concentrated sulfuric acid and preparing ammonium chloride.
The concept of reaction speed. Factors influencing the rate of reaction. Determination of reaction rate.
Demonstration. The influence of reaction conditions on its rate.
The concept of chemical equilibrium. Methods for shifting chemical equilibrium. Application of this knowledge in chemical production.
Topic 6. Combined tasks (3 hours)
Solving combined problems for different types of block C of the Unified State Exam in chemistry.
Topic 7. Qualitative reactions (3 hours)
The concept of a qualitative reaction. Identification of substances using the solubility table of acids, bases and salts, characterization of visible changes in processes. Determination of inorganic substances contained in different bottles without labels, without the use of additional reagents. Carrying out transformations of inorganic and organic substances.
Demonstration experiment. Identification of solutions of iron (II) sulfate, copper (II) sulfate, aluminum chloride, silver nitrate using sodium hydroxide solution. Identification of solutions of sodium chloride, potassium iodide, sodium phosphate, calcium nitrate using a solution of silver nitrate and nitric acid.
Carrying out a chain of transformations.
Practical work. Determination of solutions of silver nitrate, sodium hydroxide, magnesium chloride, zinc nitrate in numbered bottles without labels without the use of additional reagents.
Topic 8. Introduction to chemical analysis (6 hours)
Introduction. Chemistry, man and modern society. Introduction to chemical analysis. Fundamentals of qualitative analysis. Fundamentals of analytical chemistry. Solving typical calculation problems.
Practical work. Carrying out analysis to detect traces of blood and saliva in the samples issued. Analysis of chips and soft drinks.
Topic 9. Chemical processes (6 hours)
Characteristics of chemical processes. Chemical process, its signs. Crystals in nature. Crystallization of substances and its dependence on various factors. Chemical processes in the human body. Biochemistry and physiology.
Practical work. Crystallization of a substance. Growing crystals in the laboratory. Decomposition of hydrogen peroxide by blood enzymes.
Topic 10. Chemistry of elements (5 hours)
The essence of a chemical reaction. Solving problems involving substances of various classes and determining the type of chemical reaction. Chemical reactions that occur without changing the oxidation state of chemical elements. Reactions that occur with a change in the oxidation state of chemical elements. Ion exchange reactions.
Practical work. Precipitation of salts.
Topic 11. Corrosion of metals (3 hours)
The concept of corrosion. Signs of a corroding surface. Chemical and electrochemical corrosion. Corrosion protection.
Practical work. Techniques for protecting metal surfaces from corrosion.
Topic 12. Food chemistry (7 hours)
Chemistry and nutrition. The importance of proteins, fats and carbohydrates for complete nutrition. Factors influencing the absorption of the most important food components. Chemical characteristics of processes occurring in the digestive tract. "Live" and "dead" food. The chemistry of vegetarianism and meat-eating. Flavorings, preservatives, dyes and flavor enhancers.
Practical work. Determination of artificial colors in food. Isolation of proteins from biological objects.
Topic 13. Pharmacology (4 hours)
The concept of pharmacology. Recipe and directions. Homeopathy, its chemical bases. Contraindications and side effects, chemistry.
Practical work. The effect of antibiotics and nitrates on soil microflora.
Topic 14. Final conference: “The importance of experiment in the natural sciences” (3 hours)
From natrochthymia to chemotherapy (medicinal chemistry). Chemistry of nutritional biology. Solving typical chemical problems for passing the Unified State Exam.
Requirements for learning outcomes
In the classes of the elective course “Experimental Problems in Chemistry,” students must strictly comply with safety requirements when conducting laboratory and practical work, and know the rules of first aid for burns and poisoning with chemical reagents.
After completing the proposed course, students should:
be able to make measurements (mass of a solid using a technochemical balance, volume of a solution using a measuring cup, density of a solution using a hydrometer); prepare solutions with a given mass fraction of dissolved substance; determine the percentage concentration of solutions of acids and alkalis using the table values of their densities; plan, prepare and conduct simple chemical experiments related to dissolving, filtering, evaporating substances, washing and drying sediments; the production and interaction of substances belonging to the main classes of inorganic compounds; determination of inorganic substances in individual solutions; implementation of a chain of transformations of inorganic compounds;
solve combined problems that include elements of standard calculation problems:
determination of the mass and mass fraction of a dissolved substance in a solution obtained in different ways (by dissolving the substance in water, mixing solutions of different concentrations, diluting and concentrating the solution);
determination of the mass of the reaction product or volume of gas from the known mass of one of the reacting substances; determination of the yield of the reaction product as a percentage of the theoretically possible;
determination of the mass of the reaction product or volume of gas based on the known mass of one of the reacting substances containing a certain proportion of impurities;
determination of the mass of one of the reaction products based on the known masses of the reacting substances, one of which is given in excess.
Bibliography:
1. Gabrielyan O.S. General chemistry: tasks and exercises. M.: Education, 2006.
2. Gudkova A.S. 500 problems in chemistry. M.: Education, 2001.
3. Objectives of the All-Russian Chemistry Olympiads. M.: Exam, 2005.
4. Labiy Yu.M. Solving chemistry problems using equations and inequalities. M.: Education, 2007
5. Magdesieva N.N., Kuzmenko N.E. Learn to solve chemistry problems. M.: Education, 2006.
6. Novoshinsky I.I. Types of chemical problems and methods for solving them. M.: Onyx, 2006.
7. Okaev E.B. Chemistry Olympiads. Mn.: TetraSystems, 2005.
8. KIMs Unified State Examination in Chemistry for different years
Number
lesson
(sections, topics)
Quantity
hours
Dates
Lesson equipment
Homework
1. Introduction.
PSHE D.I.Mendeleev, portraits of scientists
Introduction.
2. Solutions and methods for their preparation
Alcohol lamp, test tube rack, test tubes, flame test wire, filter paper, evaporation dish, universal indicator paper, solutions of nitric acid, barium chloride, sodium hydroxide, lime water, silver nitrate
Mass fraction of solute.
Molar concentration and molar concentration equivalent.
Solubility of substances.
Practical work No. 1: “Preparation of a solution of a certain concentration by mixing solutions of different concentrations.”
3. Calculations using chemical equations
Alcohol lamp, stand, tongs, spatula, glass, test tubes, dropper, graduated cylinder, filter funnel, filter paper, solutions of nitric acid, silver nitrate, hydrochloric acid, D.I. Mendeleev's PSHE, solubility table, calculator
Determination of the mass of the reaction product from the known mass of one of the reactants.
Calculation of volumetric ratios of gases.
Tasks related to determining the mass of a solution.
Calculation of the mass, volume, amount of substance of the reaction product, if one of the reacting substances is given in excess.
Carrying out a reaction between substances containing known masses of reacting substances, determining the excess using an indicator.
Determination of the yield of the reaction product as a percentage of the theoretically possible.
Calculation of impurities in reacting substances.
4. Determination of the composition of mixtures
Alcohol lamp, tripod, beaker, graduated cylinder, evaporation cup, filter paper, magnesium, sulfuric acid, copper (II) oxide, magnesium carbonate, sodium hydroxide, hydrochloric acid
Determination of the composition of a mixture, all components of which interact with the specified reagents.
Determination of the composition of a mixture, the components of which selectively interact with the specified reagents.
5. Determining the formula of a substance
Derivation of the formula of a substance based on the mass fraction of elements.
Derivation of the molecular formula of a substance based on its density in hydrogen or air and the mass fraction of the element.
Derivation of the molecular formula of a substance from the relative density of its vapors and the mass, volume or amount of combustion products.
Derivation of the formula of a substance based on the general formula of a homologous series of organic compounds.
6. Patterns of chemical reactions
PSHE D.I.Mendeleev, solubility table, task cards
Calculations using thermochemical equations.
The rate of chemical reactions.
Chemical balance.
7. Combined tasks
PSHE D.I.Mendeleev, solubility table, task cards
Combined tasks.
8. Qualitative reactions
Wide test tube with gas outlet tube, stand, stopwatch, gas syringe, graduated cylinder, zinc granules and powder, dilute hydrochloric acid, hydrogen peroxide solution, manganese (IV) oxide, copper (II) oxide, zinc oxide, sodium chloride, potato slices, pieces of liver.
Methods for determining inorganic and organic substances.
Experimental determination of inorganic substances.
Experimental determination of organic substances.
34 hour
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
Ministry of Health of the Republic of Uzbekistan
Ministry of Higher and Special Education of the Republic of Uzbekistan
PRACTICUM IN GENERAL CHEMISTRY
Tashkent - 2004
Reviewers:
Professor of the Department of Bioorganic and Biological Chemistry II TashGosMI Kasymova S.S.
Assoc. Department of General Chemistry TashPMI Arifdzhanov S.Z.
A.D.Juraev, N.T.Alimkhodzhaeva and others.
Workshop on general chemistry: Textbook for medical students
The manual provides the contents of laboratory classes in the course of general chemistry for students of medical institutes. For each lesson, the goals and objectives of this topic, the issues discussed in the lesson, the significance of the topic being studied, a block of information on this topic, training tasks with standards for their solution, situational tasks, questions, tasks and tests to determine the mastery of this topic, methods of conducting laboratory tests are given. works and tasks for independent solution.
The workshop was compiled in accordance with the new program for teaching the course “General Chemistry” for students of medical institutes.
PREFACE
Chemistry is one of the fundamental general theoretical disciplines. It is closely related to other natural sciences: biology, geography, physics. Many sections of modern chemical science arose at the intersection of physical chemistry, biochemistry, geochemistry, etc. In modern chemistry, many independent sections have emerged, the most important of which are inorganic chemistry, organic chemistry, analytical chemistry, polymer chemistry, physical chemistry, etc. General chemistry examines basic chemical concepts, as well as the most important laws associated with chemical transformations. General chemistry includes the fundamentals from various sections of modern science: physical chemistry, chemical kinetics, electrochemistry, structural chemistry, etc. The most important functions of general chemistry include, firstly, the creation of a theoretical basis for the successful mastery of special disciplines, and secondly, the development of students' the process of teaching modern forms of theoretical thinking, which is extremely relevant, since among the requirements for a modern specialist, the first place is given to the need for both a theoretical view of the objects and phenomena being studied, and the ability for independent thinking, the ability to think from a scientific perspective, to go beyond the framework of a narrow specialty in solving complex problems and the acquisition of practical skills when performing analyzes of biological objects.
The role of chemistry in the medical education system is quite large. Studying such important areas in medicine as molecular biology, genetics, pharmacology, quantum biochemistry, etc. is impossible without knowledge of the theory of the structure of matter and the formation of chemical bonds, chemical thermodynamics, the mechanism of chemical reactions and other issues.
One of the sections of general chemistry according to the program for medical institutes is bioinorganic chemistry, which arose on the basis of inorganic chemistry, biochemistry, biology, and biogeochemistry.
Bioinorganic chemistry studies the composition, structure, transformation of biomolecules containing metal ions, and their modeling. This science explores the mechanisms of participation of inorganic ions in the course of biochemical processes.
Using the achievements of bioinorganic chemistry, it is possible to explain the behavior of chemical elements in biological systems.
And today the statement of the great Russian scientist M.V. Lomonosov is very true: “A physician cannot be perfect without a thorough knowledge of chemistry.”
INTRODUCTION
This textbook has been compiled to help medical students studying general chemistry. It is necessary for independent preparation of students for laboratory and practical classes.
The purpose of this manual is to, on the basis of modern achievements, develop in students the skills of qualitative and quantitative prediction of the products of transformation of substances in a living organism based on the study of typical chemical reactions, as well as systematize knowledge of the most important theoretical generalizations of chemistry; teach to apply this knowledge to phenomena occurring in a living organism in normal and pathological conditions.
As a result of mastering the course of bioinorganic chemistry:
The student should know:
The study of solutions, on the basis of which to evaluate the properties of non-electrolytes and electrolytes to predict the influence of the environment on the course of biochemical reactions (processes); ways of expressing the compositions of solutions; be guided by the protolytic theory of acids and bases as the basis for considering acid-base interactions in living organisms;
Basic concepts and laws related to the thermodynamics of chemical processes that determine the direction and depth of biochemical reactions;
Basic laws of chemical kinetics as applied to biological systems;
Basic patterns of redox processes and precipitation processes to predict the likely products of the transformation of substances in biochemical systems and drugs used in medicine;
Basic principles of the theory of structure and reactivity of complex compounds to predict the formation of the most probable products in living organisms between metal ions and bioligands for their use in medicine;
Typical properties of compounds of s, p, d elements in connection with their location in D.I. Mendeleev’s periodic table of elements for predicting the transformation of chemical elements in biological systems.
Types of chemical reactions. Exothermic and endothermic reactions
As a result of mastering the course of bioinorganic chemistry
The student must be able to:
independently work with educational and reference literature, use their data to solve typical problems as applied to biological systems;
choose reaction conditions to obtain specific compounds;
predict the possibility of chemical reactions and draw up reaction equations for their occurrence;
possess modern chemical laboratory technology for conducting qualitative and quantitative analysis of medical preparations and biological objects;
Compile abstracts for the analyzes carried out and scientifically substantiate the experimental data obtained in application to medical practice.
The manual contains the goals and objectives of this topic, the issues discussed in the lesson, the significance of the topic being studied, a block of information on this topic, training tasks with standards for their solution, which are an indicative basis for action when applying theoretical principles to specific tasks, as well as situational tasks, questions, tasks and tests to determine the mastery of this topic, methods of conducting laboratory work and tasks for independent solution.
This manual is based on works that have been used for a number of years in the educational process at the I Tashkent State Medical Institute and Tashkent PMI when studying a course in general chemistry. The workshop is compiled in accordance with the program of teaching the course, “general chemistry” for students of medical institutes.
When compiling the manual, special attention was paid to the medical bias of teaching general chemistry.
Rules for working in a chemical laboratory
The technology of modern chemical research is complex and varied. The initial stage of their implementation is laboratory practical classes in general chemistry, during which basic skills are acquired in working in a chemical laboratory with chemical equipment, glassware, etc., to perform simple experiments.
Every student working in a chemical laboratory must strictly adhere to the following work rules:
I. Each person working in the laboratory is assigned a workplace, which must not be cluttered with unnecessary objects, nor should briefcases, books, packages, etc. be placed on the table. The workplace should be kept orderly and clean.
2. Before each laboratory work, you should study the theoretical material related to it; begin experiments only after carefully reading the instructions (manual) and clarifying all unclear questions. All laboratory work must be performed individually.
3. Use reagents, gas, water, and electricity carefully. For experiments, take minimal amounts of the substance. Unused or excess reagents must not be returned to the bottles. The remains of rare, expensive and toxic compounds are poured into special vessels kept by the laboratory assistant.
4. Immediately close all bottles with reagents and solutions with stoppers that must not be mixed up after use. It is prohibited to take public reagents to your place. It is not recommended to place bottles with reagents on books and notebooks.
5. Work in the laboratory in lab coats, it is strictly forbidden to eat, and you are not allowed to smoke or talk loudly.
6. Upon completion of work, it is necessary to wash the used dishes, thoroughly clean the workplace, turn off the gas, water, and electricity.
7. All data from laboratory work performed should be recorded in a laboratory journal. It contains: theoretical material necessary to perform this work, methods of performing laboratory work, observations, reaction equations, calculations, answers to questions, solutions to problems, scientifically based results of analysis, conclusions made on the basis of the research. The entry in the journal should be accurate and compiled in such a way that a chemist who is not familiar with this work, after reading it, can clearly imagine how the experiments were carried out, what was observed in them, and what conclusions the experimenter came to. The laboratory notebook must be completed during the analysis as it is performed. The use of any drafts is not permitted. It is strictly forbidden to cover up or alter the numbers in the experimental report.
Safety rules when working in a chemical laboratory
When performing laboratory work in a chemical laboratory, safety regulations must be followed.
Laboratory work is usually carried out at a chemistry bench. The table must be clean. Before starting laboratory work, you must ensure that all reagents and glassware are available.
The experiment should be carried out strictly in the sequence indicated in its description. When heating, do not hold test tubes and flasks with the opening facing you or the person working nearby; You must not lean over the opening of the vessel in which the reaction is taking place.
Work with flammable substances away from fire.
If benzene, ether, or gasoline ignite, you cannot extinguish the fire with water; you must fill the fire with sand.
Work with caustic, toxic and odorous substances in a fume hood. Pour concentrated acids and alkalis under the draft. Under no circumstances should their remains be poured into the sink, but into specially designated bottles. Under traction, perform all reactions accompanied by the release of toxic gases or vapors.
Place hot appliances and dishes on special stands.
If you get acid on your face or hands, wash it off with a strong stream of tap water, and then rinse the affected area with a diluted solution of tea soda; If alkali gets on your skin, rinse the area thoroughly with water and then with a diluted solution of acetic acid.
If you are burned by hot objects, cover the burned area with gauze soaked in a weak solution of potassium permanganate. In case of glass cuts, the blood should be washed with a weak solution of potassium permanganate or alcohol, the wound should be lubricated with iodine solution, and bandaged.
Remember that salts containing mercury, arsenic, barium, and lead are poisonous; After using them, wash your hands thoroughly.
When testing a gas by smell, hold the test tube in your left hand so that the hole is below the level of your nose, and with your right hand direct a weak flow of air towards you.
We must remember well that in a chemical laboratory special care, conscientiousness and accuracy are required when performing laboratory work. This will ensure success at work.
Each student is allowed to conduct laboratory work only after studying the safety rules when working in a chemical laboratory.
WITHways to express the concentration of solutions in a systemSI.
Purpose of the lesson. Learn to carry out quantitative calculations to prepare solutions of various concentrations necessary for the analysis of biological objects. Learn experimentally to prepare solutions of a given concentration used in medical practice.
The significance of the topic being studied. Liquid solutions, primarily aqueous solutions, are of great importance in biology and medicine. They are the internal environment of living organisms, where vital processes take place, primarily metabolism. Biological fluids: blood plasma, lymph, gastric juice, urine, etc. are complex mixtures of proteins, lipids, carbohydrates, salts dissolved in water. The solubility of drugs in water is taken into account when using them for treatment. Solutions of medicinal products in medical practice are always used with a numerical expression of their composition. Therefore, knowledge of the units of measurement for the concentration of solutions is necessary for the doctor. Carrying out quantitative calculations for the preparation of solutions of a given concentration is very important in medical practice, since in clinical, sanitary and hygienic and other analyzes drugs are used in the form of solutions of known concentration.
Initial level of knowledge:
1.Solubility of substances in water;
2. Concepts: solute, solvent, solution;
3. Chemical theory of the formation of solutions by D.I. Mendeleev;
4. Concentration of solutions;
5. Solutions are saturated, unsaturated, supersaturated, concentrated, diluted.
N.L. Glinka. General chemistry. L., 1976, p. 213.
S.S. Olenin, G.N. Fadeev. Inorganic chemistry. M., 1979, p. 107.
A.V.Babkov, G.N.Gorshkova, A.M.Kononov. Workshop on general chemistry with elements of quantitative analysis. M., 1978, p. 32.
The following questions will be covered during the lesson::
Ways to express the concentration of solutions:
I.1. mass fraction of component - w(X), w(X)%:
I.2. mole fraction -N(X); volume fraction - f(X);
I.3. molar concentration-c(X);
I.4. molal concentration-in(X);
I.5. molar concentration of equivalent c(feq(x)x) = c(
I. 6. equivalence factor feq(x) = (
I.7. equivalent f eq(x)x = (
I.8. molar mass of equivalent M f eq(x)x = M(
I.9. amount of substance equivalent n (f eq(x)x) = n(
I.10.solution titer - t(x)
Solving problems on the topic.
3. Laboratory work
Bloc information
Basic terms and units of measurement concentrations of solutions in the SI system.
Solutions are homogeneous systems consisting of two or more components and products of their interaction. . The most significant are solutions of solid, liquid and gaseous substances in liquid solvents, usually water.
A certain amount of solute contained in a certain weight amount or a certain volume of a solution or solvent is called the concentration of the solution.
Due to the introduction of the International System of Units (SI), there have been some changes in the way the composition of a solution is expressed. In this system, the basic unit of mass, as is known, is the kilogram (kg), gram (g), the unit of volume is liter (l), milliliter (ml), the unit of quantity of a substance is the mole.
The amount of substance in the system isn(X) - a dimensional physical quantity characterized by the number of structural particles contained in a system - atoms, molecules, ions, electrons, etc. The unit of measurement for the amount of a substance is the mole. This is the amount of a substance containing as many real or conditional particles as there are atoms contained in 0.012 kg of carbon isotope with a mass of 12. For example: n(HCl) = 2 mol or 2000 mmol; n(H+)= 3?10-3 mol; n(Mg2+) = 0.03 mol or 30 mmol
Molar mass M(X) - The mass of one mole of a substance in a system is the ratio of the mass of the substance to its quantity. Units of measurement - kg/mol, g/mol.
M(X)=, g/mol
M(X)- molar mass of substance X of the system;
m(X)- mass of substance X of the system;
n(X)- amount of substance X of the system.
For example:
M(Cl2)=70.916 g/mol; M(Ca2+)=40.08 g/mol; M (NaCl) = 58.50 g/mol.
Mass fraction of the component -sch(X),sch%(X) - a relative value representing the ratio of the mass of a given component contained in a system (solution) to the total mass of this system (solution) (instead of the concept of percentage concentration). Expressed in fractions of a unit and as a percentage (%).
; ;
For example: sch %(NaCl)=20%; sch %(HCl)=37%.
Molar(molar) fraction of the component -N ( X ) - a relative value equal to the ratio of the amount of substance of a component contained in a given system (solution) to the total amount of substance of the system (solution).
The mole fraction is often denoted by the letter N(X).
Volume fraction of the component -f (X) - a relative value equal to the ratio of the volume of a component contained in a system (solution) to the total volume of the system (solution).
Molar concentration -s(X) the ratio of the amount of substance (X) in a system (solution) to the volume of this system (solution).
With (X)= =, mol/l
With (NSl)= 0.1 mol/l; c(Cu2+)= 0.2378 mol/l
Molal concentration -b(x) - the ratio of the amount of substance (X) contained in the system (solution) to the mass of the solvent.
V(x) = mol/kg
For example
in(NSl)= 0.1 mol/kg.
Equivalence factor- f eq(X)= - a dimensionless quantity indicating what fraction of a real particle of a substance (X) is equivalent to one hydrogen ion in an acid-base reaction or one electron in a redox reaction. The equivalence factor is calculated based on the stoichiometry of a given reaction. For example:
NaOH+H2SO4=Na2SO4+H2O ; f eq(NaOH)=1, feq(H2SO4 )=
Equivalent -f eq(X) - dimensionless quantity - a real or conditional particle of a substance (X), which in a given acid-base reaction combines with one mole of hydrogen or is in some way equivalent to it or equivalent to one electron in redox reactions.
Molar mass equivalent -M( f eq(x)) = M the mass of one mole equivalent of a substance, equal to the product of the equivalence factor and the molar mass of the substance:
M(f eq(x)x) = M() = f eq(x)MM(x), g/mol
M(H2SO4) = M(H2SO4) = 49.0 g/mol
TOamount of substance equivalent
n ( f eq( x ) x ) = n (
- the amount of a substance in which the particles are equivalent to:
n(= , mole; n(Ca2+)= 0.5 mol
Molar concentration equivalent
With( f eq(x)x)=c(
- the ratio of the amount of an equivalent substance in a system (solution) to the volume of this system (solution):
With(feq(x)x)= s= =mol/l = 0.1 mol/l
Solution titer -t ( x )- mass of substance (X) contained in 1 ml of solution:
t (x) = - ,g/ml
t(HCl)= 0.003278 g/ml
Training tasks and standards for their solution.
m(H2 O)=200.00g
m(CuSO4·5Н2О) =50.00g
M(CuSO4)=342.16g/mol
M(CuSO4·5Н2О)=25000 g/mol
sch%(CuSO4·5H2O)=?
sch% (CuSO4)=?
Solution reference
Find the mass of the resulting solution:
m(p- p)= m(in-in)+m(H2 O)=50.00 g+200.C g=250.00 g.
m(p-p)=250.00G.
Find the mass fraction of CuSO4 5H2O in the solution:
sch% (CuSO4 5H2O) =
sch%( CuSO4 5H2O)=
We find the mass of anhydrous salt in 50.00 g of copper sulfate. The molar mass of CuSO4 5H2O is 250.00 g/mol, the molar mass of CuSO4 is 160.00 g/mol. One mole of CuSO4·5H2O contains one mole of CuSO4. Thus, I mol x 250.00 g/mol = 250.00 g CuSO4 5H2O contains I mol x 160.00 g/mol = 342.16 g CuSO4:
in 250.00 g CuSO4 5H2O -160.00 g CuSO4
We make up the proportion: 250.00: 160.00 = 50.00: x.
Solving it, we find the mass of anhydrous copper sulfate:
Find the mass fraction of anhydrous salt:
sch%( CuSO4)=
sch%( CuSO4)=
sch%( CuSO4·5Н2О)=20%;sch%( CuSO4) = 25,60%
Task No. 2 How many ml of 96% (mass) solution of H2SO4 (c = 1.84 g/ml) should be taken to prepare 2 liters of 0.1000 mol/l solution of H2SO4?
sch%(H2SO4)=96%;
With=1.84g/ml
V(p- p)=2.00l
With(H2 SO4)=0.1000 mol/l
M(H2SO4)=98.0g/mol
V(H2SO4)=?
Solution reference
1. Find the mass of H2SO4 containing in 2 liters of solution a molar concentration of 0.1000 mol/l. It is known that
With(H2 SO4)= , Then
m(H2SO4)= c(H2 SO4) M(H2SO4) V(p- p)
m(H2SO4)=0,1000 M98 M2,00 G
m(H2SO4)=19.60g.
2. Find the mass of a 96% (mass) H2SO4 solution containing 19.60 g of H2SO4
sch%(H2SO4)=
m(p- p)=
3. Find the volume of the H2SO4 solution, knowing its density.
m(p- p)= V(p- p) MWith (p- p); Then V(p- p)=
V(p- p)= 20.42/1.84=11.10ml
V(H2 SO4)= 11.10ml
Task No. 3. Determine the molar concentration of 200 g of antiseptic 2.0% (wt.) alcohol solution of brilliant green (“green”). M(brilliant green) = 492 g/mol; (c=0.80g/ml).
sch%(in-va)=2.0%
With(solution)=0.80g/ml
M(v-v)=492.0g/mol
s(in-in)=?
Solution standard.
Find the mass of the substance in 200.00 g of brilliant green solution.
Find the volume of the alcohol solution:
V(p-p)=V(p-p)=
Find the molar concentration of c(v) in solution:
s(in-in)=s(in-in)=
s(in)=0.06500mol/l
Task No. 4. The titer of NaOH solution, widely used in drug analysis, is 0.003600 g/ml. When reacting with sulfuric acid, it forms an acid salt. What is the molar concentration of the equivalent solution in its reaction with sulfuric acid; mass fraction of NaOH(%) in solution? Calculate the amount of NaOH required to prepare 1 liter of such a solution.
t(NaOH) =0.003800 g/ml
V(p- p)=1.00 l
M(NaOH)=40.0 g/mol
With (p- p)=1.0g/ml
With(NaOH)=?m(NaOH)=?
sch%(NaOH)=?
Solution standard.
The equation for the reaction that occurs is:
H2SO4 + NaOH = Na HSO4 + H2O
feq(H2SO4)=1; feq(NaOH)=1.
Thus, in this case we should talk about the molar concentration of the NaOH solution.
Find the mass of NaOH required to prepare 1000 ml of solution:
t(NaOH)=
m(NaOH)= t(NaOH)V(p-p)
m(NaOH)=0.003800 1000gml/ml=3.8g
Find the molar concentration of the solution:
With(NaOH)=
With(NaOH)==0.0950mol/l
Find the mass of 1 liter of solution:
m(solution)=1000ml 1 g/ml=1000g
4. Find the mass fraction of NaOH (%) in the solution:
sch%(NaOH)=
sch%(NaOH)=
Answer: With(NaOH)=0.0950mol/l
sch%(NaOH)= 0,38%
m(NaOH)=3.8g
Situational tasks.
1. How many ml of 30% (wt.) solution of HCl (c = 1.152 g/ml) should be taken to prepare 1 liter of 3% (wt.) of its solution, used internally in case of insufficient acidity of gastric juice? What is the molar concentration and titer of the resulting solution. (The solution is standardized by NaOH).
Answer: V(HCl)=84.60ml; c(HCl) = 0.8219 mol/l.
2. Calculate the molar concentration of physiological NaCl solution. How much water should be added to 200 ml of 20% NaCl solution (=1.012 g/ml) to prepare 5 L of saline?
Answer: c (NaCl) = 0.000147 mol/l
V(H2O) = 4504 ml
3. Nicotinic acid - vitamin PP - plays a significant role in the life of the body, being a prostatic group of a number of enzymes. Its deficiency leads to the development of pellagra in humans. Ampoules for medicinal purposes contain 1 ml of 0.1% (wt.) nicotinic acid. Determine the molar concentration of the equivalent and the titer of this solution
Standardization is carried out using NaOH solution.
Answer: t(H-R)=0.00100g/ml
c(H-R)=0.08130 mol/l
Test questions
Calculate the equivalence factor of Н2S04 in this reaction
Н2S04+KOH = KHS04 + H2O
a) 1b) 2c) 1/2d) 1/3e) 3
The titer of the NaOH solution is 0.03600 g/ml. Find the molar concentration of this solution.
a) 9 mol/l b) 0.9 mol/l c) 0.09 mol/l d) 0.014 mol/l e) 1.14 mol/l
Which solution does the V solubility value refer to?< V кристаллизация.
a) saturated solutionc) supersaturated solution
b) unsaturated solution d) dilute solution
d) concentrated solution
Find the mass fraction (%) of glucose in a solution containing 280 g of water and 40 g of glucose
a) 24.6% b) 12.5% c) 40% d) 8% e) 15%
Determine the equivalence factor of H2SO4 in this reaction
Mg(OH)2+2H2SO4=Mg(HSO4)2+2H2O
a) 2 b) 1 c) 1/2 d) 4 d) 3
The molal concentration of a substance in solution is determined by:
a) molar number of the substance in 1 liter of solution
b) molar number of the substance in 1 ml of solution
c) molar number of the substance in 1 kg of solution
d) molar number of the substance in 1 g of solution
How many types of aggregative states of a solution are there?
a) 2b) 3c) 1 d) 4
9. Specify the concentrated solution of NaOH:
a) 0.36% b) 0.20% c) 0.40% d) 36%
Find the molar concentration of physiological NaCl solution.
n% (NaCl)=0.85%
a) 1 mol/l b) 0.14 mol/l c) 1.5 mol/l e) 9.31 mol/l d) 10 mol/l
LABORATORY WORK 1
1.1 Preparation of solutions of a given concentration
There are three methods for preparing a solution of a given concentration:
diluting a more concentrated solution
use of a certain weight of solid matter.
method of using fixanal.
1. Preparation of a 0.1 molar solution of sulfuric acid by diluting more than concentrated solution:
Pour a solution of sulfuric acid into a beaker and use a hydrometer to determine the density of this solution. Then, using the table, determine the mass fraction of sulfuric acid in this solution.
Measure the required volume of sulfuric acid in a small beaker and carefully use a funnel to pour it into a 100 ml volumetric flask half filled with distilled water. Cool the mixture in the volumetric flask to room temperature and carefully add water to the measuring mark. Close the volumetric flask tightly with a lid and, after thorough mixing, hand it over to the laboratory assistant.
Preparation of the solution by dissolving a certain portion of a solid:
Ask your teacher what concentration of solution you need to prepare. Then carry out the calculation: how many grams of salt need to be dissolved to obtain a solution with a given concentration and weigh the required amount of salt with an accuracy of 0.01 g.
Stir the solution with a glass rod with a rubber tip until the salt is completely dissolved. If an increase or decrease in temperature is observed during the dissolution process, wait until the solution reaches room temperature.
Pour the resulting solution into a dry cylinder and use a hydrometer to measure the density of the resulting solution. Using the table, determine the mass fraction of the dissolved substance corresponding to the density.
% error = (shteor-schpractic) · 100/shteor
INveintroduction to titrimetric analysis
Purpose of the lesson: To become familiar with the basics of titrimetric analysis, as one of the quantitative research methods used in medical practice for the analysis of biological objects and medications, as well as for sanitary assessment of the environment.
The significance of the topic being studied. The method of titrimetric (volume) analysis is widely used in biomedical research to determine the quantitative composition of biological objects, medicinal and pharmacological preparations.
Without knowledge of the composition of various environments of living organisms, neither understanding the essence of the processes occurring in them, nor the development of scientifically based treatment methods is possible. Diagnosis of many diseases is based on comparing test results for a given patient with the normal content of certain components in the blood, urine, gastric juice, and other body fluids and tissues. Therefore, medical professionals, especially doctors, need to know the basic principles and methods of titrimetric analysis.
Initial level of knowledge.
Fundamentals of the theory of electrolytic dissociation of acids, bases, salts;
Types of chemical reactions (in molecular and ionic form);
Methods of expressing the concentration of solutions.
Educational material for self-study.
1. V.N. Alekseev. Quantitative analysis. M., 1972, p. 193.
2. A.A.Seleznev. Analytical chemistry. M., 1973, p. 164.
I.K. Tsitovich. Analytical chemistry course. M., 1985, p.212.
The lesson will cover the following questions:
1. Problems of analytical chemistry
2. The essence of titrimetric analysis methods
2.1. Basic concepts: solutions used in titrimetric analysis
2.2. Equivalence point
2.3. Requirements for reactions used in titrimetric analysis
2.4. Measuring glassware: burettes, pipettes, volumetric flasks, graduated cylinders.
2.5. Titration technique.
2.6. Calculations using the titrimetric method
2.7. Classification of titrimetric analysis methods
Application of titrimetric analysis methods in medical practice.
4. Laboratory work
Information block
Analytical chemistry is a science that studies methods for determining the qualitative and quantitative chemical composition of substances or their mixtures. It is divided into qualitative and quantitative analysis. Qualitative analysis methods are used to determine which chemical elements, atoms, ions or molecules the analyzed substance consists of. Quantitative analysis methods are used to establish the quantitative ratios of the constituent components of a given compound under study.
Quantitative analysis is carried out using various methods. Chemical methods are widespread in which the amount of a substance is determined by the amount of reagent spent on titration, by the amount of sediment, etc. The most important are three methods: gravimetric, titrimetric (volumetric) and colorimetric.
The essence of the gravimetric analysis is that the component of the analyzed substance is completely isolated from the solution in the form of a precipitate, the latter is collected on a filter, dried, calcined in a crucible and weighed. Knowing the weight of the resulting sediment, the content of the desired component is determined using the chemical formula of the latter.
In titrimetric (volumetric) analysis, the quantitative determination of the constituent components of the analyte is carried out by accurately measuring the volume of a reagent of known concentration that enters into a chemical reaction with the analyte.
The colorimetric method of analysis is based on comparing the color intensity of the test solution with the color of a solution whose concentration is precisely known.
In clinical analysis, titrimetric analysis methods are most widely used, since they do not require much time, are easy to perform, and can be used to obtain fairly accurate results.
The titrimetric analysis method is based on the precise measurement of the volume of reagent consumed in the reaction with the analyte X. The process of adding one solution in a burette to another solution to determine the concentration of one of them (with a known concentration of the other) is called titration. The term titration is derived from the word titer, which means the content of the reagent in grams in 1 ml of solution.
A solution of a reagent of precisely known concentration is called a working titrated or standard solution. A solution with a precisely known concentration can be obtained by dissolving an exact sample of a substance in a known volume of solution or by determining the concentration using another solution, the concentration of which is known in advance. In the first case, a solution with a prepared titer is obtained, in the second - with a set titer.
To prepare a solution with a given concentration, only those substances are suitable that can be obtained in very pure form, have a constant composition, and do not change in air or during storage. These substances include many salts (sodium tetraborate Na2B4O7 10H2O, sodium oxalate Na2C2O4, potassium dichromate K2Cr2O7, sodium chloride NaCl); oxalic acid H2C2O4 2H2O and some others. Substances that meet the listed requirements are called initial or standard.
Accurate determination of the concentration of working solutions is one of the main prerequisites for obtaining good results of volumetric analysis. Carefully prepared and tested working solutions are stored under conditions that prevent changes in the concentration of the solution due to evaporation, decomposition of the substance or contamination from the environment. The concentration of working solutions is periodically checked using standard solutions.
To prepare titrated solutions, you can also use commercially available fixatives. These are glass ampoules containing precisely weighed quantities of various solids or precisely measured volumes of liquids necessary to prepare 1 liter of solution with the exact molar concentration equivalent. To prepare a solution from fixanal, the contents of the ampoule are transferred to a 1-liter volumetric flask, after which the substance is dissolved and the volume is adjusted to the mark.
During titration, it is necessary to establish the end point of the reaction, i.e. the point of equivalence when the quantities of reactants in a mixture become equivalent. For this purpose, titrimetric analysis uses indicators. Indicators are substances that are added in small quantities to solutions during titration and change color at the equivalence point.
To determine the moment of equivalence, in addition to color, changes in other properties of the solution can be used, but this requires physicochemical measurements. The latter are increasingly used in volumetric analysis.
In titrimetric analysis, only those reactions are used that satisfy the following conditions:
the interaction between the analyte and the reagent must occur in certain stoichiometric ratios;
the reaction between the analyte and the reagent must proceed at high speed;
the chemical reaction between the analyte and the reagent must proceed completely, i.e. Reversibility of the reaction is not allowed;
the reaction between the analyte and the reagent should not be accompanied by any side reactions.
To accurately measure volumes, measuring utensils are used: burettes, pipettes, volumetric flasks and graduated cylinders.
Burettes are designed for titration and accurate measurement of the volume of consumed reagent. These are graduated glass tubes, the lower end of which is tapered and equipped with either a ground glass stopcock or a rubber tube with a ball-type stopper connected to a pipette. Burettes are made with a capacity of 10 to 100 ml. For particularly accurate analyses, 1 and 2 ml microburettes are used. The most commonly used burettes are with a capacity of 10 to 50 ml. The graduation of the burette begins at the top, from there large divisions of 1 ml go down to the bottom mark. Whole milliliters are divided into tenths. The volume of liquid poured from the burette is determined by the difference in levels before and after titration. Liquid level readings must be carried out very accurately. The accuracy of readings is hampered by the fact that the burette has a concave meniscus. The visible shape of the meniscus depends on the lighting conditions, so white paper should be placed closely behind the burette when taking measurements. When counting, the eyes should be at the level of the meniscus. Burettes are filled using a funnel. The top of the burette is covered with a cap to prevent dust from getting into it. Before filling with the solution, the burette must be rinsed three times with the same solution.
Pipettes are used in cases where it is necessary to measure a certain exact volume of liquid from a prepared solution and transfer it to another vessel. Pipettes are glass tubes with a widening in the middle and a slight narrowing at the lower end. The pipette capacity is indicated on the top. Pipettes are manufactured with a capacity from 1 ml to 100 ml. Graduated pipettes have divisions of 25, 10, 5, 2, 1 ml. Micropipettes of 0.2 and 0.1 ml are also used to measure thousandths of a milliliter. Pipettes are stored in special racks in a vertical position. Fill the pipette with the solution using a rubber bulb or draw the solution into the pipette with your mouth through the top of the tube. The latter method is not recommended due to the possibility of liquid getting into the mouth. When filling the pipette with a solution, suck the latter slightly above the mark and then quickly clamp the upper hole with your index finger so that the liquid does not spill out of the pipette. The filled pipette is raised slightly so that the tip comes out only of the solution, but not from the vessel from which the solution is taken. Then, keeping the eye at the level of the mark, carefully release the pressure of the finger, slightly lifting its end, and the liquid flows out drop by drop. As soon as the lower part of the meniscus reaches the mark line, the pipette hole is tightly closed with a finger and the measured liquid is poured into another vessel. Draining the solution from the pipette is done by touching the tip of the pipette to the wall of the vessel into which the solution is poured. Typically, allow the solution to drain freely or slow down the rate of draining by covering part of the top opening of the pipette with your finger. When all the liquid has poured out, you need to wait 20 - 30 seconds, then remove the pipette from the vessel. The droplet of liquid remaining on the tip of the pipette should not be blown out, as this was taken into account when calibrating the pipette. When working with a pipette, before filling the latter with a solution, it is necessary to rinse the pipette several times with the same solution.
After finishing work, the pipette should be rinsed with distilled water.
Volumetric flasks are used mainly for preparing solutions of a certain concentration. These are flat-bottomed vessels with a narrow and long neck. There is a mark on the neck in the form of a ring, up to which you need to fill the flask (along the lower edge of the liquid meniscus) in order to obtain the volume indicated on the wide part of the flask. Volumetric flasks are designed for volumes of 50, 100, 200, 500, 1000, 5000 ml. The capacity of the flask is indicated in the inscription on the flask. The flask is closed with a ground glass stopper. Fill the flask first through a funnel inserted into it, and then from a pipette so that the lower meniscus is opposite the line.
Graduated cylinders are used to measure specific volumes of solutions when accuracy is not of great importance. They are convenient for mixing and diluting solutions of a certain volume. There are divisions along the height of the cylinder. When measuring, the eye should always be level with the lower meniscus. Measuring cylinders are not used to accurately measure volumes.
Glassware intended for performing chemical analyzes must be thoroughly washed. This is one of the most important elements of the job to ensure accurate results. The criterion for the cleanliness of glassware is the flow of water droplets from the inner walls. If drops appear on the walls during rinsing, then before starting work, you need to wash the dishes again. You can use special brushes. After this, the dishes are filled with a chrome mixture, which oxidizes traces of organic substances on the glass, and kept for some time (up to half an hour). After washing the dishes, the chrome mixture is collected for reuse. After pouring the chrome mixture into a collection bottle, the dishes are rinsed first with tap water and then with distilled water. If the dishes must be used dry, they are dried in special drying cabinets.
Titration is carried out as follows:
A clean burette is rinsed 2-3 times with a small amount of working solution to remove residual water.
Fix the burette vertically in the tripod leg and fill it with the titrated solution to a level slightly above zero.
Part of the solution is lowered into the supplied glass to displace air from the rubber tube and pipette.
Bring the liquid level to zero. There should not be a drop of solution left on the tip of the burette (it is removed by touching the glass).
The test solution is pipetted into the titration flask.
Gradually pour the liquid from the burette into the flask until the equivalence point is established.
When reading fluid, the eye is held exactly at the level of the meniscus. For colored solutions, the reading is made along the upper meniscus, for uncolored solutions - along the lower one.
At the end of the work, the burette is filled with water above the zero division and closed on top with a test tube.
During chemical analyses, errors may occur, so several parallel measurements are carried out. Systematic errors in titrimetric analysis can arise due to incorrect determination of the concentration of working solutions, changes in concentration during storage, inaccuracy of volumetric glassware, incorrect choice of indicator, etc.
The source of random errors is: inaccuracy in filling the burette to zero division, inaccuracy in reading the volume on the burette scale, uncertainty in the excess of the reagent after adding the last drop of the working solution during titration.
Calculations in titrimetric analysis are carried out according to law of equivalents: at the same molar concentrations of the equivalent, the solutions interact with each other in equal volumes. At different concentrations, the volumes of solutions of interacting substances are inversely proportional to their concentrations:
V1 s(1/z X1) = V2 s(1/z X2) (1)
For both reactants, the product of the molar concentration of the equivalent of its solution and the volume is a constant value. Based on the law of equivalents, various quantitative calculations can be carried out.
For example, knowing the molar concentration of the equivalent of one solution, as well as the volumes of solutions spent on titration, you can determine the molar concentration and titer of another solution. For example:
To neutralize 20.00 ml of sulfuric acid solution, 12.00 ml of alkali solution with a molar concentration equivalent to 0.2000 mol/l was consumed. Calculate the molar concentration of the equivalent and the titer of sulfuric acid in this solution.
2 NaOH + H2SO4 = Na2SO4 + 2 H2O
NaOH + S H2SO4 = S Na2SO4 + H2O
From the equation it is clear that the equivalence factor of H2SO4 is equal to ½, and the equivalence factor of NaOH is equal to 1. Substituting the values into formula (1) we obtain:
c(S H2SO4) = 0.2000 mol/l · 12.00 ml / 20.00 ml = 0.1200 mol/l
t(Н2SO4) = с(1/2 H2SO4) · M(1/2 H2SO4) / 1000, g/ml
Hence t(H2SO4) = 0.1200 mol/l 49 g/m/1000 = 0.005880 g/mol
Calculations in titrimetric analysis must be carried out with a high degree of accuracy.
The volumes of solutions are measured accurate to hundredths of a milliliter, for example: V (HCI) = 10.27 ml or V (NaOH) = 22.82 ml. The concentration of solutions is calculated to the fourth significant figure, for example:
c(NSI)=0.1025 mol/l
c (NaOH)=0.09328 mol/l
t(NSI) = 0.003600 g/ml
Depending on the reaction that underlies the determination, methods of volumetric analysis can be divided into the following groups:
Acid-base titration methods or neutralization method
Oxidation-reduction or oxidimetry methods
Complexometry method
Precipitation methods
Educational tasks and standards and their solutions
Task No. 1. In medicine, potassium permanganate is used as an antiseptic externally for washing wounds and throat - 0.1-0.5% solution, for gargling - 001 - 01% solution, for gastric lavage - 0.02 - 0.1% solution. Which titrimetric analysis method can be used to calculate the concentration of a potassium permanganate solution if a titrated solution of oxalic acid is available?
Solution reference
Potassium permanganate is an oxidizing agent, oxalic acid is a reducing agent. Since the reaction between these components is redox, the permanganatometry method can be used to determine the concentration of potassium permanganate.
Task No. 2. Determine the molar concentration of the equivalent and the titer of hydrogen chloride if 19.87 ml of 0.1 mol/l NaOH solution was used to titrate 20.00 ml of this solution.
V(HCl)= 20.00 ml
V(NaOH)= 19.87 ml
c(NaOH)= 0.1000 mol/l
M(HCl) = 36.5 g/mol
c(HCl) = ?t(HCl) = ?
Solution standard.
The equation for the reaction that occurs is:
NaOH + HCl = NaCl + H2O
Thus: f eq (NaOH) = 1, f eq (HCl) = 1.
Using the law of equivalents, we find the molar concentration of the HCl solution:
c(NaOH) V(NaOH) = c(NSl) V(HCl)
c(HCl) =mol/l
Based on the value of c(HCl), we calculate the titer of this solution:
t(HCl) =
t(HCl)= 0.003627g/ml
Answer: c(HCl) = 0.09935 mol/l
t(HCl) = 0.003627 g/ml
Situational tasks.
Answer: V(NaOH) = 12.33 ml.
2. In what cases does the equivalence point lie at pH=7, at pH<7, при рН>7?
Answer: When titrating a strong acid with an alkali, the equivalent point coincides with the neutral point; when titrating a weak acid with an alkali, the equivalent point lies at the pH values<7, при титровании слабого основания сильной кислотой эквивалентная точка лежит выше нейтральной точки.
3. Lead acetate - Pb(CH3COO)2 - is an astringent for inflammatory skin diseases. A 0.5% solution is used. Calculate the mass of this substance to prepare 100 ml of a 0.5% (mass) solution. What is the mass fraction of lead (%) in this solution? p=1 g/ml.
Answer: m(Pb(CH3COO)2 = 0.5 g w% = (Pb) = 0.32%.
Test questions.
1. What value of solution titer t(HCl) reflects the required degree of accuracy of determinations in titrimetric analysis
a) 0.03 g/ml b) 0.003715 g/ml c) 0.0037578 g/ml) 3.7 g/ml d) 0.0037 g/ml
2. What volume values are consistent in titrimetric analysis?
a) 2.51 ml; 10.52 ml; 8.78 ml d) 15.27 ml; 15.22 ml; 15.31 ml
b) 5.73 ml; 7.02 ml; 15.76 ml c) 1.07 ml; 5.34 ml; 0.78 ml.
3. What measuring utensil is used to determine the volume of the titrated solution?
a) pipette c) volumetric flask b) burette c) flask
4. What reaction is the basis of acid-base titration?
a) redox reaction
b) neutralization reaction
c) reaction of formation of complex compounds
d) a reaction that occurs with the release of heat
5. Which solution is called titrated?
a) solution of unknown concentration
b) freshly prepared solution
c) a solution of a reagent of precisely known concentration
d) a solution whose concentration needs to be determined
6.What is an equivalence point?
a) this is the end point of the reaction b) this is the starting point of the reaction
c) interaction of two substances d) point where the volumes are equal
7.What law are the calculations based on in titrimetric analysis?
a) law of conservation of mass of matter b) law of equivalents
c) Ostwald's law of dilution d) Raoult's law
8. For what purpose are pipettes used?
a) for measuring the exact volume of solution b) for titration
c) for preparing solutions d) for diluting a solution
9. What is the titer of a solution?
a) this is the number of grams of dissolved substance in 1 liter of solution
b) this is the number of moles of dissolved substance in 1 liter of solution
c) this is the number of moles of solute in 1 kg of solution
d) this is the number of grams of solute in 1 ml of solution
10.What substances are used to determine the equivalence point?
a) indicators b) inhibitors c) promoters d) catalysts
LABORATION WORK 2
2.1 Techniques for working with laboratory measuring glassware used in titanium rimetric analysis (on water)
...Similar documents
Basic concepts of chemical thermodynamics. Standard enthalpy of combustion of a substance. Corollaries from Hess's law. The role of chemistry in the development of medical science and practical healthcare. Elements of chemical thermodynamics and bioenergetics. Thermochemistry.
presentation, added 01/07/2014
The essence and subject of analytical chemistry as a science. Tasks and methods of qualitative and quantitative analysis of chemical substances. Examples of qualitative reactions to cations. Characteristics of the phenomena accompanying reactions by wet (in solutions) and dry routes.
presentation, added 04/27/2013
Application of qualitative analysis in pharmacy. Determination of authenticity, testing for purity of pharmaceuticals. Methods for performing analytical reactions. Working with chemical reagents. Reactions of cations and anions. Systematic analysis of the substance.
tutorial, added 03/19/2012
Origin of the term "chemistry". Main periods of development of chemical science. Types of the highest development of alchemy. The period of the birth of scientific chemistry. Discovery of the basic laws of chemistry. Systems approach in chemistry. The modern period of development of chemical science.
abstract, added 03/11/2009
Theoretical basis of analytical chemistry. Spectral methods of analysis. The relationship of analytical chemistry with sciences and industries. The meaning of analytical chemistry. Application of precise methods of chemical analysis. Complex metal compounds.
abstract, added 07/24/2008
The main stages of the development of chemistry. Alchemy as a phenomenon of medieval culture. The emergence and development of scientific chemistry. Origins of chemistry. Lavoisier: revolution in chemistry. Victory of atomic-molecular science. The origins of modern chemistry and its problems in the 21st century.
abstract, added 11/20/2006
The concept of refraction as a measure of the electronic polarizability of atoms, molecules, ions. Refractive index assessment for identification of organic compounds, minerals and medicinal substances, their chemical parameters, quantitative and structural analysis.
course work, added 06/05/2011
The potentiometric method is a method of qualitative and quantitative analysis based on measuring the potentials that arise between the test solution and the electrode immersed in it. Potentiometric titration curves.
test, added 09/06/2006
"Assay art" and the history of the emergence of laboratories. Creative development of Western European chemical science. Lomonosov M.V. as an analytical chemist. Russian achievements in the field of chemical analysis in the 18th-19th centuries. Development of domestic chemistry in the 20th century.
course work, added 10/26/2013
From alchemy to scientific chemistry: the path of real science about the transformations of matter. Revolution in chemistry and atomic-molecular science as the conceptual basis of modern chemistry. Environmental problems of the chemical component of modern civilization.
The folder contains materials that will help organize the practical part of chemistry for children with disabilities and distance learning
Download:
Preview:
To use the preview, create a Google account and log in to it: https://accounts.google.com
Preview:
MONITORING THE ACHIEVEMENT OF PLANNED RESULTS IN A CHEMISTRY COURSE (FROM WORK EXPERIENCE)
Dushak Olga Mikhailovna
Regional budgetary educational institution "School of Distance Education", Zheleznogorsk,
Key words: new Federal State Educational Standard, planned results, chemistry, ongoing monitoring, microskills
Annotation: The article describes the experience of using such forms of control as the Feedback Sheet and the Sheet of Achievement of Planned Results in the Chemistry course for grades 8-9.
The teacher’s activities within the framework of the new educational standard are result-oriented. The planned educational outcome, prescribed in the Federal State Educational Standard, is differentiated. The planned results of mastering the curriculum are presented in two blocks: “The graduate will learn” (basic level) and “The graduate will have the opportunity to learn” (advanced level). On the FIPI website, teachers and students can familiarize themselves with measurement materials for the final certification of students. To successfully pass the final certification, the student must master a system of concepts, subject knowledge and skills. The teacher is faced with the task of developing this knowledge and skills, creating a system for assessing the achievement of planned results during ongoing monitoring. Having studied the materials of the new Federal State Educational Standard, methodological literature, and the experience of my colleagues, I began to create my own system for tracking the effectiveness of achieving the planned results when studying the topics of the Chemistry course for grades 8-9. As the basis for the classification, I took the system considered by A.A. Kaverina, senior researcher. Center for Natural Science Education, Institute for Education Development Strategy, Russian Academy of Education, Ph.D.
To assess the achievement of planned results, it is necessary to develop criteria. The criteria must be developed correctly, accessible and reflect the gradual assimilation of knowledge and skills to create comfortable conditions for the child to acquire cognitive experience, his advancement from the zone of actual development to the zone of proximal development and beyond. During the last academic year, I developed and tested algorithms for completing tasks, feedback sheets, achievement sheets for some sections of the Chemistry course in grades 8-9.
During the educational process, at the beginning of studying each topic, students are offered a list of concepts for the final test and criteria for assessing their educational results in the form of skills and micro-skills, reflected in the Feedback Sheets and assignments for them. During the study of the topic, the results are noted in the List of Achievements. Assignments can be used both when studying a new topic and when consolidating and generalizing educational material. For example, in the Section on the Variety of Chemical Reactions, the following skills are developed: to compose equations for the electrolytic dissociation of acids, alkalis, and salts; compose complete and abbreviated ionic equations for exchange reactions. The feedback sheet that the student receives contains micro-skills for step-by-step completion of the task, which is also attached. To evaluate my own results, I offer students a simple scale: I can + I can’t-.
Task No. 1 Create salt formulas using the valence values for the metal and the acid residue; name the substances, write the dissociation equation (the text of the task is given in the form of a fragment).
Acids | Metals | Dissociation equation for one salt |
||||
Fe(II) | Fe(III) |
|||||
Name | ||||||
HNO3 | ||||||
Name | ||||||
Evaluation criteria: I can + I can’t -
Task No. 2 Create formulas for the proposed substances, determine the class, write dissociation equations for these substances: potassium chloride, silver nitrate, sodium carbonate, magnesium sulfate, lead nitrate, potassium sulfide, potassium phosphate (the text of the task is given as a fragment).
Feedback sheet________________________________________________F.I.
Topic: Ionic equations BASIC LEVEL!
I can: DATES: | Test |
|||
Draw up formulas of complex substances by valence | ||||
Define class | ||||
Name the substance | ||||
Write the equation of dissociation of matter |
Evaluation criteria: I can + I can’t -
Task No. 3 Write equations for exchange reactions between the proposed pairs of substances. Equalize, compose complete and abbreviated ionic equations (the text of the task is given in the form of a fragment).
Feedback sheet_____________________________________________F.I.
Topic: Ionic equations BASIC LEVEL!
I can: DATES: | Test |
|||
Write the products of metabolic reactions | ||||
Set odds | ||||
Identify substances that are not subject to dissociation | ||||
Write the complete ionic equation | ||||
Write the abbreviated ionic equation |
Evaluation criteria: I can + I can’t -
After successfully completing basic-level tasks, the student gets the opportunity to complete advanced-level tasks, which indicates the formation of the ability to apply acquired knowledge to solve educational and educational-practical problems in a changed, non-standard situation, as well as the ability to systematize and generalize the acquired knowledge.
For example, when completing task No. 3 onelevated level, the student can formulate a conclusion about in which case the ion exchange reactions proceed to completion. Using the Table of Solubility of Acids, Bases and Salts, create examples of molecular equations for the given abbreviated ionic: Ba 2+ + SO 4 2- = BaSO 4 ; CO 3 2- + 2H + = H 2 O + CO 2, etc.
This organization of the educational process has shown a number of advantages: the possibility of an individual trajectory when mastering a topic, criteria for assessing the results of work that are understandable to the child and his parents. In the future, we plan to continue working on developing assignments for other sections of the course.
Bibliography:
1. Kaverina A.A. Chemistry. Planned results. Task system. 8-9 grades: a manual for teachers of general education institutions / A.A. Kaverina, R.G. Ivanova, D.Yu. Dobrotin; edited by G.S. Kovaleva, O.B. Loginova. – M.: Education, 2013. – 128 p. – (We work according to new standards)
Preview:
Grade 8 Practical work on the topic:Soil and water analysis
Experience 1
Mechanical soil analysis
In a test tube (or vial) Place the soil (the soil column should be 2-3 cm). Add distilled water(boiled), the volume of which should be 3 times the volume of the soil.
Close the test tube with a stopper and shake thoroughly for 1-2 minutes, then use a magnifying glass and observe the sedimentation of soil particles and the structure of sediments. Describe and explain your observations.
Experience 2
Preparation of soil solution and experiments with it
Prepare paperfilter (or from cotton wool, bandage), insert it into the funnel attached to the tripod ring. Place a clean, dry test tube under the funnel and filter the mixture of soil and water obtained in the first experiment. The mixture should not be shaken before filtering. The soil will remain on the filter, and the filtrate collected in the test tube is a soil extract (soil solution).
Place a few drops of this solution on a glass plate and, using tweezers, hold it over the burner until the water evaporates(just leave it on the battery).What are you observing? Explain.
Take two litmus papers (red and blue)(if there is!), Apply the soil solution to them with a glass rod. Draw a conclusion based on your observations:
1. After the water evaporates on the glass………..
2. Universal litmus paper will not change its color if the solution is neutral, it will turn red if it is acidic, and blue if it is alkaline.
Experience 3
Determination of water clarity
For the experiment you need a transparent flat-bottomed glass cylinder(tumbler) diameter 2-2.5 cm, height 30-35 cm You can use a 250 ml measuring cylinder without a plastic stand. INDICATE THE SIZES OF YOUR GLASS
We recommend conducting the experiment first with distilled water and then with water from a pond and comparing the results. Place the cylinder on the printed text and pour in the water to be tested, making sure that the text can be read through the water. Note at what height you will not see the font. Measure the heights of the water columns with a ruler. Draw conclusions:
The measured height is called the visibility level.
If the visibility level is low, then the reservoir is heavily polluted.
Experience 4
Determining the intensity of the odor of water
Conical flask(jar) fill 2/3 volume of the test water, close tightly with a stopper (preferably glass) and shake vigorously. Then open the flask and note the character and intensity of the odor. Give an assessment of the intensity of the water odor in points using Table 8.
Use table 8 (page 183).
MAKE A GENERAL CONCLUSION
Preview:
Section V Experimental Chemistry
- When performing a chemical experiment, identify signs indicating the occurrence of a chemical reaction
- Conduct experiments to recognize aqueous solutions of acids and alkalis using indicators
Related concepts:
Chemical phenomenon (reaction), experiment, acid, alkali, signs of a chemical reaction, solution, indicators
Signs of a chemical reaction:
Change in color, odor, precipitation or dissolution of precipitate, release of gas, release or absorption of heat and light
Task No. 1
Feedback sheet__________________________________________F.I.
Topic: Experimental chemistry. Signs of chemical reactions
I can: DATES: | Test |
|||
Follow the rules for working with substances | ||||
Record changes that occur with substances during the experiment | ||||
Identify signs of a chemical reaction | ||||
Record observations | ||||
Write the reaction equation in molecular form | ||||
Formulate a conclusion |
Evaluation criteria: I can + I can’t -
Experience name | Video length, email address | Signs of a reaction | Reaction equation |
|
Interaction of acids with metals | 37 sec | |||
Reaction between copper oxide and sulfuric acid | 41 sec |
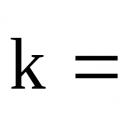 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe