Why is the water in the sea salty? Traditional hypothesis. We study the salinity of the seas: why is the water in the sea salty? Why is the water fresh?
Water occupies most of the Earth's territory. The vast majority of water is found in oceans and seas, and it is salty. According to the Ocean Service, ocean water contains more than 3% sodium chloride (common salt).
Why is the water in the seas and oceans salty and where does this salt come from? We will try to figure out the answer to this question in the article.
General information
It happened that sailors of ships that got lost in sea waters or were shipwrecked died of thirst, although there was a lot of water around. Few people know that sea water has a composition that is not suitable for the human body. It has a specific bitter-salty taste, which is imparted by salts dissolved in water.
Rivers flowing into the seas have fresh water, in which the concentration of dissolved salts is much lower than in sea water. But how is this possible, why is the water in the sea salty and the water in the river fresh?
For 4 billion years, the continents have been watered by rain. Water penetrates the rocks and finds its way to the sea. She carries dissolved salt with her. Over the course of a long geological history, the amount of salt gradually increases. This is one of the simplest hypotheses.
A little about the benefits and harms of salt
Before we find out why the sea is salty but the rivers are not, let’s decide whether salt is beneficial or harmful. As it turned out, there are huge reserves of salt on Earth, both in the seas (sea) and in the bowels of the earth (rock). It has been scientifically proven that sodium chloride is a vital substance. People have long known that salt is a fairly useful and valuable substance, necessary for both people and animals.
However, there is also a negative side: excessive salt in the soil leads to a decrease in its fertility. As a result, desertification occurs (for example, in Australia).

Why is the water in the sea salty?
Some of the salt gets into the water from the seabed, which contains salt-containing rocks, from which salt gets into the water. Sodium chloride can also come from volcanic valves. But most of the salt comes from the continents. One kilogram of sea water on average contains up to 35 grams of salt, and the majority (about 85%) is sodium chloride (the well-known kitchen salt).
Sources of salt entering the sea:
- Weathering of rocks: when rocks get wet, substances are washed away from them, and salts are carried into the sea (a similar effect occurs with rocks on the seabed).
- Explosions of underwater volcanoes: they release lava into the water, which reacts with sea water and dissolves some substances in it.
Water has the ability to penetrate into cracks that lie deep in the ocean in the mid-ocean ridge zones. The rocks there are hot (there is often lava at the bottom). Water, heating up in cracks, dissolves a large amount of salts from underwater rocks, which fall into sea water.

Why is the water in the sea salty? Because sodium chloride is the most common salt in it due to the fact that it dissolves better than all substances. However, silicon and calcium are also carried by rivers to the oceans in large quantities. However, there are not so many of them in sea water. This is due to the fact that calcium is “picked up” by various aquatic animals (corals, gastropods and bivalves), and silicon is used by microscopic algae (cell walls are created).
The sun causes the evaporation of huge amounts of water in the seas and oceans. The evaporated water leaves behind salt, which concentrates in the sea. Therefore, the water becomes salty. It should be noted that some salt is deposited on the seabed. Thanks to this, the balance of salt concentration in the water is maintained, otherwise the sea would become even more salty.

Are the versions true?
Where does the salt in the sea come from? Which hypothesis is the most correct? No version can be considered the most correct. The water in the sea and ocean was formed over millions of years, so scientists do not have reliable evidence of its salinity. It is known that water washes away mainland soil, which does not contain as much salt. The salinity of water changed in different geological eras. And each sea has a different concentration of salt and different properties. The density of water varies, and there are differences in freezing points.
It turns out that everyone knows the fact of salinity, but the exact cause of this phenomenon remains a mystery.
Some factors affecting the concentration of salt in water
When answering the question why the water in the sea is salty, one should also find out why the salt concentration of different seas differs. The salinity of water varies depending on the location of the natural reservoir. The least salty oceans and seas are located closer to the north and south poles, where the sun does not shine much, and therefore the water does not evaporate. In addition, the water is diluted with water from melted glaciers.

Sea waters near the equator evaporate more due to elevated temperatures. This factor also influences the increased density of water in these places. This process can also occur in some large lakes, which also turn into salt lakes. An example is the Dead Sea, where the density and salinity of the water allows people to lie calmly on its surface.
The sea water temperature also affects the salt concentration. The Baltic Sea can be cited as an example. Due to the low water temperatures, it contains 8 times less salt than, for example, in the Persian Gulf.
Finally
The above hypotheses for the cause of water salinity in the seas and oceans are the opinion of scientists at the current level of knowledge.
One interesting fact. If water from all the oceans and seas existing on Earth were to evaporate today, the remaining salt would form a layer up to 75 meters high around the world.
At school they ask quite a lot of interesting questions. Some of them at first glance seem quite simple and easy to answer, although in fact everything is far from so simple. Tell me, do you know why the water in the sea is salty? We strongly doubt this, since even scientists do not know the exact answer!
Versions and hypotheses
Let's start, perhaps, with this - when did water bodies on Earth become salty? This probably happened a long time ago. But when exactly? Some historians claim that this happened millions of years ago, even before the dinosaurs became extinct. Others are sure that some time ago the seas consisted exclusively of fresh water... Now you can’t tell who is right and who is wrong.
- But let's return to our main question. According to the school curriculum, water bodies became salty thanks to rivers. But how can this be, you ask, because the water in the rivers is fresh! We will agree with you, but we will add that it also contains dissolved salts, albeit in microscopic quantities. Nevertheless, they are there, although we cannot taste them. Based on this, it turns out that rivers not only desalinate the seas, but also salinize them. After river water enters the sea water, a certain part of it evaporates under the influence of the natural environment, but the salts do not disappear anywhere and remain in the sea. Scientists have even found out that it is thanks to rivers that the World Ocean receives almost three million tons of a wide variety of substances and elements. Huge number! Imagine that such a cycle in nature has been going on for more than one million years? Then it’s clear why the water in some reservoirs is so salty...
It would seem that the answer has been found. But wait! Other experts who support other theories say that almost all salts that fall into the sea precipitate and over time, huge rock layers and rocks begin to form from them. In addition, river and sea water contain very different substances and elements. So, in the first there is negligible amount of table salt, but there is a lot of carbonates, lime and soda, and the second is known for a large amount of table salt and sodium. In general, not everything is so obvious.
- The second theory on this issue is also very interesting. Those experts who support it argue that over the past several billion years that our planet has existed, the rivers have always been fresh and the seas have always been salty. Theoretically, in this case, river water could become salty, but the laws of nature intervene here - seas and oceans cannot flow into rivers, this happens exactly the opposite even in our time.
- According to the third version, animals played a significant role. Thus, one of the scientists claims that once upon a time river water was practically no different from sea water. Many animals used it for drinking. If you haven't forgotten, it contains a large amount of calcium, which is so necessary for the development of the skeleton of living creatures. So, the animals gradually fished out from the rivers all the elements they needed, among which were salts. This happened over hundreds of millions of years, as a result of which the rivers practically got rid of sodium chloride. Of course, this theory has a right to life, although it sounds very far-fetched. Why? It's simple - the reserves of sea salt are simply huge. So, if it is evenly distributed over land, it will cover our entire planet with a layer more than a hundred meters thick! Can you imagine that fish and animals could eat so much mineral, even over a huge period of time? We doubt it.
- This theory is supported by many experts. They say it's all the volcanoes' fault. When the earth's crust first began to form, there was enormous volcanic activity on Earth. Gases from volcanoes contained vapors of fluorine, bromine and chlorine, so acid rain occurred periodically. It was they who formed the seas, which, of course, were also acidic. However, this water reacted chemically with hard rocks, extracting from them alkaline elements such as sodium, potassium, magnesium and calcium. This is how salts formed, which neutralized the acidity of the water, gradually making it salty. The composition of the water finally stabilized about 500 million years ago.
Bottom line
But there is no result as such, because neither we nor scientists know the answer to the question posed. But we still hope that someday a specialist will be able to solve this mystery of nature.
Geography
Natural science
The world
Why is the sea salty?
“Why is the sea salty?” - one of children's favorite summer questions. In our new column “Why” we will regularly answer the most interesting questions of preschoolers and schoolchildren in clear and simple language, as well as hold exclusive competitions!Why is the sea salty? Why does a hedgehog need needles? Why did they add “-s” to many words in the last century? Why do cats purr and what do they do? Is it possible to create a time machine according to the laws of physics? As a parent or teacher of primary and secondary schools, you will hear these questions more than once. We will be happy to answer them.
Why is the sea salty?
The answer to this question must begin with an explanation of where the water in the sea and ocean comes from. In rivers we find springs and springs - underground springs, but where does the water, and salty one, come from in the sea?
The reserves of both the Black Sea and the Atlantic Ocean are replenished with fresh water from rivers and precipitation in the form of snow or rain. Both consist of fresh water (in fact, also salty, just in a very small concentration). But unlike rivers, water from oceans and seas does not flow anywhere, but only evaporates when exposed to the sun’s rays. When evaporation occurs, the salts remain.
Another factor in the salinity of the sea is the movement of the rivers themselves flowing into it. On the way to the seas and oceans, river flows wash the salts that make up the stone out of the rocks and bring them with them to the sea, albeit in small quantities.
It turns out that the sea has become salty? Was it fresh before that? No, that's not true. The main reason, which modern scientists agree with, is the process of formation of the sea itself, which was just as salty millions of years ago. The fault for this is not the rivers, which did not exist then, but the volcanoes that covered our planet.
The water of the primary ocean was formed from volcanic gases, the composition of which is approximately the following: 75% of water accounts for 15% carbon dioxide and about 10% of various chemical compounds. These compounds include methane, ammonia, sulfur, chlorine and bromine, as well as various gases. So when the products of the eruption fell to the ground in the form of acid rain, they reacted with the bottom of the future sea, and as a result we got a salty solution.
How much salt is there in the sea?
About 35 are dissolved in one liter of sea water grams of salt.
How much water is there in the sea?
If we take the average depth of the world's oceans to be 3703 meters, and take the average surface area to be 361.3 million square kilometers, we get 1.338 billion km 3Which seas are the freshest and saltiest?
Let's start with another record holder - the largest sea. The absolute champion in this category is the Sargasso Sea, which is located inside the Atlantic Ocean. Its area reaches 8.5 million square kilometers.
But the freshest sea is in Russia, and this sea is the Baltic. Compared to the waters of the Atlantic, its sunshine is 5 times lower. Why? About 250 rivers flow into the Baltic Sea, which “desalinize” the waters.
What about the saltiest sea?
The record holder for the percentage of salts is the Red Sea. Its salinity is about 41 grams per liter of water! This phenomenal content explains the unique properties of the sea: it is very easy to float in it, and being in it itself is quite beneficial for health.
Why is the Red Sea so salty? The point is the fumes, which we wrote about at the very beginning. Water evaporates from this sea at a tremendous speed due to high temperature and low humidity, so that rains simply do not have time to “desalinate” it, and besides, very little of it falls.
Question - competition
Using the data above, calculate how much TOTAL salt is dissolved in ALL the seawater on our planet?
Send your answers in private messages to our communities at
Why is the water in the sea salty? There is so much water on the Earth's surface that it is often called the "blue planet." Land occupies only 29% of the Earth's area, and the remaining 70% falls on the mysterious and almost unexplored oceans. Obviously, such a quantity of water cannot have an absolutely identical composition, as can be seen from the example of the different saturation of salts in rivers and seas. But how to explain these differences?
Water is famous for its ability to erode any type of rock. It doesn’t matter what sharpens the stone - a powerful stream or a separate drop - the result is always predictable. During the destruction of the rock, it removes easily soluble components from it. Salts, which are also leached from the stone, give the water its characteristic taste.
Scientists have not been able to come to a consensus as to why some bodies of water have fresh water and others have salt water. To date, two complementary theories have been formulated.
First theory
The first theory is based on the fact that fresh water is just as salty as sea water, but the concentration of salt in it is seventy times lower. Salt-free water can only be obtained in laboratory conditions by distillation, while natural liquids have never been and will not be purified from chemical components and microorganisms.
All impurities that dissolve and are then washed away by water from rivers and streams inevitably end up in the waters of the World Ocean. Then the water evaporates from its surface and turns into, and the salt becomes part of its chemical composition. This cycle has been continuously repeated for two billion years, so it is not surprising that during this time the World Ocean has become so rich in salts.
Proponents of this theory cite salt lakes that have no drainage as evidence. If the water did not initially contain a sufficient amount of sodium chloride, they would be fresh.
Sea water has one unique property: it contains almost all existing chemical elements, including magnesium, calcium, sulfur, nickel, bromine, uranium, gold and silver. Their total number is close to sixty. However, the highest level is due to sodium chloride, also known as table salt, which is responsible for the taste of sea water.
And it was the chemical composition of water that became the stumbling block to this hypothesis. According to research, sea water contains a high percentage of hydrochloric acid salts, while river water contains carbonic acid salts. The question of the reason for such differences still remains open.
Second theory
The second point of view is based on the assumption of the volcanic nature of ocean salts. Scientists believe that the process of formation of the earth's crust was accompanied by increased volcanic activity, as a result of which gases saturated with fluorine, boron and chlorine vapors were transformed into acid rain. From this we can conclude that the first seas on Earth contained a huge percentage of acid.
Under such conditions, living organisms could not originate, but subsequently the acidity of ocean water decreased significantly, and it happened like this: acidic water washed out alkalis from basalt or granite, which were then transformed into salts that neutralized ocean water.
Over time, volcanic activity weakened significantly, and the atmosphere began to gradually clear itself of gases. The composition of sea water also stopped changing and reached a stable state five hundred million years ago.
However, even today the salinity of the water is controlled by a large number of underwater volcanoes. When they begin to erupt, the minerals in the lava mix with the water, raising the overall salt level. But, despite the fact that a new portion of various salts enters the World Ocean every day, its own salinity remains unchanged.
Returning to the question of carbonates disappearing from fresh water when it enters the sea, it is worth adding that these chemicals are actively used by marine organisms to form shells and skeletons.
Everyone knows that sea water is very harmful and tastes unpleasant. However, many adhere to the erroneous ideas that it can easily replace fresh water in conditions of extreme necessity. Such misconceptions can not only harm a person who finds himself in an extreme situation, but also cost him his life.

The thing is that the load associated with filtering any liquid entering the body falls entirely on the kidneys. Their task is to remove excess fluid through urine and sweat. In the case of sea water, the kidneys will have to process a large amount of salts, which can be retained, forming stones and impairing the functioning of the entire body.
Thanks to the kidneys, during the day a person excretes about fifty percent of the liquid he drinks during this period. Instead, excess sodium, calcium and potassium salts leave the body with urine. Sea water is so saturated with salt that the kidneys wear out very quickly, trying to cope with work that is too much for them. One liter of sea water contains thirty-five grams of salt, which is several times higher than its content in human water.
The daily fluid intake for an adult includes not only water, but also moisture received during meals. Every day, from fifteen to thirty-five grams of salt deposits in the body, which the kidneys successfully remove.
Thus, it turns out that in order to get rid of thirty-five grams of salt that entered the body along with a liter of sea water, it will have to produce one and a half liters of its own fluid, taking into account the fact that the amount of water drunk will clearly not be enough for this. To fulfill their task, the kidneys will begin to work to the limit of their capabilities and very quickly fail.
In addition, a lack of fluid coupled with a critical level of salt in the body will lead to severe dehydration, and after a few days the kidneys will stop functioning. Excess salt will cause damage to internal organs, the first of which will be the kidneys and gastrointestinal tract. Due to a lack of moisture, irreversible changes will also occur in the nervous system.
In addition, dehydration in the process of quenching thirst with sea water is caused by the presence of magnesium sulfate in its composition, which has a laxative effect. As a result, dehydration occurs much more rapidly than usual, and the person quickly loses strength and ability to fight for survival.
The body can no longer produce its own fluid and cope with high salt levels. In addition, sea water contains other dangerous substances, on the absorption of which the body will spend its last resources.
However, it is still possible to survive in the absence of fresh water. Some scientists and survival experts advise squeezing the liquid out of fish, no matter how strange it may sound. There are several documented cases where people managed to escape with the help of such fish “juice”.
Thus, the salt contained in the waters of the World Ocean can bring people both the sensation of flight from swaying on the surface of the sea, and become their worst enemy, gradually depriving them of the ocean that is contained in the body of each of us.
Did you know that sailors lost in sea waters most often died of thirst? This is a paradox - after all, the ship is surrounded by thousands of tons of life-giving moisture! The fact is that the chemical composition of sea water is not suitable for our body, so it cannot be drunk. In addition, it has a specific taste - due to the salts dissolved in it. The question arises: how did they get there and why is the water in the sea salty?
Ocean waters contain almost all elements of the periodic table. Most of all - hydrogen and oxygen, which are combined into water molecules. There are also impurities containing:
- calcium;
- magnesium;
- bromine;
- sulfur;
- fluorine.
But the main mineral part is made up of chlorine and sodium ions, that is, ordinary salt, which gives the water a salty taste. It remains to be seen who salted the water in the seas.
How sea water was formed
Scientists still have not found an answer to the question of why sea water is salty and river water is not. There are two hypotheses for the formation of sea water. The main difference between them is the way they look at the beginning of this process. Some believe that the ocean became salty quite recently, while others are sure that this happened in the early stages of the planet’s existence.
River infusions
The waters of rivers and lakes are also salty. But we don’t feel this, since the sodium chloride content in them is 70 times less than in the sea. According to the “river” hypothesis of the origin of sea water, dissolved impurities enter the ocean with the flow of rivers. The water in the sea gradually evaporates, but the minerals remain, so their concentration is constantly increasing. The process of ocean salinization, according to this group of scientists, has been going on for several billion years, resulting in water becoming more and more salty.
However, studies conducted over many years show that the salt content in the world's oceans does not change for a long time, and substances entering it with river water can only maintain this value at the same level. In addition, this hypothesis does not explain the different composition of river and sea water: rivers have a lot of carbonates, while chlorides predominate in the sea.
Consequence of volcanic activity
Proponents of the second hypothesis believe that sea water was salty already when life on Earth did not yet exist. And the reason for this is volcanoes. During the formation of the earth's crust, many magma emissions occurred. Volcanic gases contained compounds of bromine, fluorine and chlorine, which fell as part of acid rain. As a result, an acidic ocean appeared on the planet.
The ocean's acids began to react with the alkaline elements of the hard rocks of the earth, giving rise to more stable compounds - salts. Thus, table salt, which is familiar to us, was formed as a result of the interaction of perchloric acid from the ocean and sodium ions from frozen volcanic rocks.
Gradually, the sea water became less acidic and acquired a salty taste. Proponents of this theory believe that the ocean acquired its modern properties 500 million years ago, when the Earth’s surface was cleared of volcanic gases and the composition of the water stabilized.
Then how to explain the disappearance of carbonates that come with the river flow? This is the “work of the hands” of marine inhabitants. They learned to use these minerals to build skeletons and shells, which are necessary for the protection and mechanical support of the body.
In which sea is it impossible to drown?
The salts that make up water can change its properties, including density. The higher it is, the more difficult it is to immerse a solid body in liquid, so it is easier to swim in sea water. From this point of view, many are interested in which sea has the saltiest water.
The Dead Sea, which is actually a lake and is fed by the waters of the Jordan River, has the highest concentration of sodium chloride. It is located between Israel and Jordan and is very attractive to tourists who want to relax and improve their health. Most of all, people like to swim there, since the high density of the water prevents drowning.
The saltiest water in the world has a salinity index of 33.7%, which is almost 9 times higher than that in the world's oceans. This sea was called dead due to the absence of its usual inhabitants - algae and fauna. But many types of microscopic organisms live in it - fungi, omycetes and bacteria.
Why is the sea salty: Video
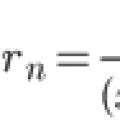 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A