Iron scale with concentrated sulfuric acid. Sulfur(VI) oxide
Task No. 1
Sodium was heated in a hydrogen atmosphere. When water was added to the resulting substance, gas evolution and the formation of a clear solution were observed. Brown gas was passed through this solution, which was obtained as a result of the interaction of copper with a concentrated solution of nitric acid. Write equations for the four reactions described.
1) When sodium is heated in a hydrogen atmosphere (T = 250-400 o C), sodium hydride is formed:
2Na + H 2 = 2NaH
2) When water is added to sodium hydride, an alkali NaOH is formed and hydrogen is released:
NaH + H 2 O = NaOH + H 2
3) When copper reacts with a concentrated solution of nitric acid, brown gas is released - NO 2:
Cu + 4HNO 3 (conc.) = Cu(NO 3) 2 + 2NO 2 + 2H 2 O
4) When brown gas NO 2 is passed through an alkali solution, a disproportionation reaction occurs - nitrogen N +4 is simultaneously oxidized and reduced to N +5 and N +3:
2NaOH + 2NO2 = NaNO3 + NaNO2 + H2O
(disproportionation reaction 2N +4 → N +5 + N +3).
Task No. 2
Iron scale was dissolved in concentrated nitric acid. A sodium hydroxide solution was added to the resulting solution. The resulting precipitate was separated and calcined. The resulting solid residue was fused with iron. Write equations for the four reactions described.
The formula of iron scale is Fe 3 O 4.
When iron scale interacts with concentrated nitric acid, iron nitrate is formed and nitrogen oxide NO 2 is released:
Fe 3 O 4 + 10HNO 3 (conc.) → 3Fe (NO 3) 3 + NO 2 + 5H 2 O
When iron nitrate reacts with sodium hydroxide, a precipitate is released - iron (III) hydroxide:
Fe(NO 3) 3 + 3NaOH → Fe(OH) 3 ↓ + 3NaNO 3
Fe(OH) 3 is an amphoteric hydroxide, insoluble in water, decomposes when heated into iron (III) oxide and water:
2Fe(OH) 3 → Fe 2 O 3 + 3H 2 O
When iron(III) oxide fuses with iron, iron(II) oxide is formed:
Fe 2 O 3 + Fe → 3FeO
Task No. 3
The sodium was burned in air. The resulting substance was treated with hydrogen chloride when heated. The resulting simple yellow-green substance, when heated, reacted with chromium (III) oxide in the presence of potassium hydroxide. When a solution of one of the resulting salts was treated with barium chloride, a yellow precipitate formed. Write equations for the four reactions described.
1) When sodium is burned in air, sodium peroxide is formed:
2Na + O 2 → Na 2 O 2
2) When sodium peroxide reacts with hydrogen chloride when heated, Cl 2 gas is released:
Na 2 O 2 + 4HCl → 2NaCl + Cl 2 + 2H 2 O
3) In an alkaline environment, chlorine reacts when heated with amphoteric chromium oxide to form chromate and potassium chloride:
Cr 2 O 3 + 3Cl 2 + 10KOH → 2K 2 CrO 4 + 6KCl + 5H 2 O
2Cr +3 -6e → 2Cr +6 | . 3 - oxidation
Cl 2 + 2e → 2Cl − | . 1 - recovery
4) A yellow precipitate (BaCrO 4) is formed by the interaction of potassium chromate and barium chloride:
K 2 CrO 4 + BaCl 2 → BaCrO 4 ↓ + 2KCl
Task No. 4
Zinc is completely dissolved in a concentrated solution of potassium hydroxide. The resulting clear solution was evaporated and then calcined. The solid residue was dissolved in the required amount of hydrochloric acid. Ammonium sulfide was added to the resulting clear solution and the formation of a white precipitate was observed. Write equations for the four reactions described.
1) Zinc reacts with potassium hydroxide to form potassium tetrahydroxocinate (Al and Be behave similarly):
2) After calcination, potassium tetrahydroxozincate loses water and turns into potassium zincate:
3) Potassium zincate, when reacting with hydrochloric acid, forms zinc chloride, potassium chloride and water:
4) Zinc chloride, as a result of interaction with ammonium sulfide, turns into insoluble zinc sulfide - a white precipitate:
Task No. 5
Hydroiodic acid was neutralized with potassium bicarbonate. The resulting salt reacted with a solution containing potassium dichromate and sulfuric acid. When the resulting simple substance reacted with aluminum, a salt was obtained. This salt was dissolved in water and mixed with a solution of potassium sulfide, resulting in the formation of a precipitate and the release of gas. Write equations for the four reactions described.
1) Hydroiodic acid is neutralized by the acid salt of weak carbonic acid, resulting in the release of carbon dioxide and the formation of NaCl:
HI + KHCO 3 → KI + CO 2 + H 2 O
2) Potassium iodide enters into a redox reaction with potassium dichromate in an acidic environment, while Cr +6 is reduced to Cr +3, I - is oxidized to molecular I 2, which precipitates:
6KI + K 2 Cr 2 O 7 + 7H 2 SO 4 → Cr 2 (SO 4) 3 + 4K 2 SO 4 + 3I 2 ↓ + 7H 2 O
2Cr +6 + 6e → 2Cr +3 │ 1
2I − -2e → I 2 │ 3
3) When molecular iodine reacts with aluminum, aluminum iodide is formed:
2Al + 3I 2 → 2AlI 3
4) When aluminum iodide reacts with a solution of potassium sulfide, Al(OH) 3 precipitates and H 2 S is released. The formation of Al 2 S 3 does not occur due to complete hydrolysis of the salt in an aqueous solution:
2AlI 3 + 3K 2 S + 6H 2 O → 2Al(OH) 3 ↓ + 6KI + 3H 2 S
Task No. 6
Aluminum carbide was completely dissolved in hydrobromic acid. A solution of potassium sulfite was added to the resulting solution, and the formation of a white precipitate and the evolution of a colorless gas were observed. The gas was absorbed by a solution of potassium dichromate in the presence of sulfuric acid. The resulting chromium salt was isolated and added to the barium nitrate solution, and the formation of a precipitate was observed. Write equations for the four reactions described.
1) When aluminum carbide is dissolved in hydrobromic acid, a salt is formed - aluminum bromide, and methane is released:
Al 4 C 3 + 12HBr → 4AlBr 3 + 3CH 4
2) When aluminum bromide reacts with a solution of potassium sulfite, Al(OH) 3 precipitates and sulfur dioxide is released - SO 2:
2AlBr 3 + 3K 2 SO 3 + 3H 2 O → 2Al(OH) 3 ↓ + 6KBr + 3SO 2
3) By passing sulfur dioxide through an acidified solution of potassium dichromate, Cr +6 is reduced to Cr +3, S +4 is oxidized to S +6:
3SO 2 + K 2 Cr 2 O 7 + H 2 SO 4 → Cr 2 (SO 4) 3 + K 2 SO 4 + H 2 O
2Cr +6 + 6e → 2Cr +3 │ 1
S +4 -2e → S +6 │ 3
4) When chromium (III) sulfate reacts with a solution of barium nitrate, chromium (III) nitrate is formed, and white barium sulfate precipitates:
Cr 2 (SO 4) 3 + 3Ba(NO 3) 2 → 3BaSO 4 ↓ + 2Cr(NO 3) 3
Task No. 7
Aluminum powder was added to the sodium hydroxide solution. Excess carbon dioxide was passed through the solution of the resulting substance. The precipitate that formed was separated and calcined. The resulting product was fused with sodium carbonate. Write equations for the four reactions described.
1) Aluminum, as well as beryllium and zinc, is capable of reacting with both aqueous solutions of alkalis and anhydrous alkalis during fusion. When aluminum is treated with an aqueous solution of sodium hydroxide, sodium tetrahydroxyaluminate and hydrogen are formed:
2) When carbon dioxide is passed through an aqueous solution of sodium tetrahydroxoaluminate, crystalline aluminum hydroxide precipitates. Since, according to the condition, an excess of carbon dioxide is passed through the solution, it is not carbonate that is formed, but sodium bicarbonate:
Na + CO 2 → Al(OH) 3 ↓ + NaHCO 3
3) Aluminum hydroxide is an insoluble metal hydroxide, therefore, when heated, it decomposes into the corresponding metal oxide and water:
4) Aluminum oxide, which is an amphoteric oxide, when fused with carbonates, displaces carbon dioxide from them to form aluminates (not to be confused with tetrahydroxoaluminates!):
Task No. 8
Aluminum reacted with sodium hydroxide solution. The released gas was passed over heated copper (II) oxide powder. The resulting simple substance was dissolved by heating in concentrated sulfuric acid. The resulting salt was isolated and added to the potassium iodide solution. Write equations for the four reactions described.
1) Aluminum (also beryllium and zinc) reacts with both aqueous solutions of alkalis and anhydrous alkalis when fused. When aluminum is treated with an aqueous solution of sodium hydroxide, sodium tetrahydroxyaluminate and hydrogen are formed:
2NaOH + 2Al + 6H 2 O → 2Na + 3H 2
2) When hydrogen is passed over heated copper (II) oxide powder, Cu +2 is reduced to Cu 0: the color of the powder changes from black (CuO) to red (Cu):
3) Copper dissolves in concentrated sulfuric acid to form copper (II) sulfate. In addition, sulfur dioxide is released:
4) When copper sulfate is added to a solution of potassium iodide, an oxidation-reduction reaction occurs: Cu +2 is reduced to Cu +1, I - is oxidized to I 2 (molecular iodine precipitates):
CuSO 4 + 4KI → 2CuI + 2K 2 SO 4 + I 2 ↓
Task No. 9
We carried out electrolysis of a sodium chloride solution. Iron (III) chloride was added to the resulting solution. The precipitate that formed was filtered and calcined. The solid residue was dissolved in hydroiodic acid. Write equations for the four reactions described.
1) Electrolysis of sodium chloride solution:
Cathode: 2H 2 O + 2e → H 2 + 2OH −
Anode: 2Cl − − 2e → Cl 2
Thus, as a result of its electrolysis, gaseous H 2 and Cl 2 are released from a sodium chloride solution, and Na + and OH − ions remain in the solution. In general, the equation is written as follows:
2H 2 O + 2NaCl → H 2 + 2NaOH + Cl 2
2) When iron (III) chloride is added to an alkali solution, an exchange reaction occurs, as a result of which Fe(OH) 3 precipitates:
3NaOH + FeCl 3 → Fe(OH) 3 ↓ + 3NaCl
3) When iron (III) hydroxide is calcined, iron (III) oxide and water are formed:
4) When iron (III) oxide is dissolved in hydroiodic acid, FeI 2 is formed, while I 2 precipitates:
Fe 2 O 3 + 6HI → 2FeI 2 + I 2 ↓ + 3H 2 O
2Fe +3 + 2e → 2Fe +2 │1
2I − − 2e → I 2 │1
Task No. 10
Potassium chlorate was heated in the presence of a catalyst, releasing a colorless gas. By burning iron in an atmosphere of this gas, iron oxide was obtained. It was dissolved in excess hydrochloric acid. To the resulting solution was added a solution containing sodium dichromate and hydrochloric acid.
1) When potassium chlorate is heated in the presence of a catalyst (MnO 2, Fe 2 O 3, CuO, etc.), potassium chloride is formed and oxygen is released:
2) When iron is burned in an oxygen atmosphere, iron scale is formed, the formula of which is Fe 3 O 4 (iron scale is a mixed oxide of Fe 2 O 3 and FeO):
3) When iron scale is dissolved in excess hydrochloric acid, a mixture of iron (II) and (III) chlorides is formed:
4) In the presence of a strong oxidizing agent - sodium dichromate, Fe +2 is oxidized to Fe +3:
6FeCl 2 + Na 2 Cr 2 O 7 + 14HCl → 6FeCl 3 + 2CrCl 3 + 2NaCl + 7H 2 O
Fe +2 – 1e → Fe +3 │6
2Cr +6 + 6e → 2Cr +3 │1
Task No. 11
Ammonia was passed through hydrobromic acid. A solution of silver nitrate was added to the resulting solution. The precipitate that formed was separated and heated with zinc powder. The metal formed during the reaction was exposed to a concentrated solution of sulfuric acid, which released a gas with a pungent odor. Write equations for the four reactions described.
1) When ammonia is passed through hydrobromic acid, ammonium bromide is formed (neutralization reaction):
NH 3 + HBr → NH 4 Br
2) When solutions of ammonium bromide and silver nitrate are combined, an exchange reaction occurs between the two salts, resulting in the formation of a light yellow precipitate - silver bromide:
NH 4 Br + AgNO 3 → AgBr↓ + NH 4 NO 3
3) When silver bromide is heated with zinc powder, a substitution reaction occurs - silver is released:
2AgBr + Zn → 2Ag + ZnBr 2
4) When concentrated sulfuric acid acts on metal, silver sulfate is formed and a gas with an unpleasant odor is released - sulfur dioxide:
2Ag + 2H 2 SO 4 (conc.) → Ag 2 SO 4 + SO 2 + 2H 2 O
2Ag 0 – 2e → 2Ag + │1
S +6 + 2e → S +4 │1
Task No. 12
9С278С
Chromium(VI) oxide reacted with potassium hydroxide. The resulting substance was treated with sulfuric acid, and an orange salt was isolated from the resulting solution. This salt was treated with hydrobromic acid. The resulting simple substance reacted with hydrogen sulfide. Write equations for the four reactions described.
1) Chromium (VI) oxide CrO 3 is an acidic oxide, therefore, it reacts with alkali to form a salt - potassium chromate:
CrO 3 + 2KOH → K 2 CrO 4 + H 2 O
2) Potassium chromate in an acidic environment is converted without changing the oxidation state of chromium into dichromate K 2 Cr 2 O 7 - an orange salt:
2K 2 CrO 4 + H 2 SO 4 → K 2 Cr 2 O 7 + K 2 SO 4 + H 2 O
3) When treating potassium dichromate with hydrobromic acid, Cr +6 is reduced to Cr +3, and molecular bromine is released:
K 2 Cr 2 O 7 + 14HBr → 2CrBr 3 + 2KBr + 3Br 2 + 7H 2 O
2Cr +6 + 6e → 2Cr +3 │1
2Br − − 2e → Br 2 │3
4) Bromine, as a stronger oxidizing agent, displaces sulfur from its hydrogen compound:
Br 2 + H 2 S → 2HBr + S↓
Task No. 13
Magnesium powder was heated in a nitrogen atmosphere. When the resulting substance interacted with water, a gas was released. The gas was passed through an aqueous solution of chromium(III) sulfate, resulting in the formation of a gray precipitate. The precipitate was separated and treated by heating with a solution containing hydrogen peroxide and potassium hydroxide. Write equations for the four reactions described.
1) When magnesium powder is heated in a nitrogen atmosphere, magnesium nitride is formed:
2) Magnesium nitride is completely hydrolyzed to form magnesium hydroxide and ammonia:
Mg 3 N 2 + 6H 2 O → 3Mg(OH) 2 ↓ + 2NH 3
3) Ammonia has basic properties due to the presence of a lone electron pair on the nitrogen atom and, as a base, enters into an exchange reaction with chromium (III) sulfate, as a result of which a gray precipitate is released - Cr(OH) 3:
6NH3. H 2 O + Cr 2 (SO 4) 3 → 2Cr(OH) 3 ↓ + 3(NH 4) 2 SO 4
4) Hydrogen peroxide in an alkaline environment oxidizes Cr +3 to Cr +6, resulting in the formation of potassium chromate:
2Cr(OH) 3 + 3H 2 O 2 + 4KOH → 2K 2 CrO 4 + 8H 2 O
Cr +3 -3e → Cr +6 │2
2O − + 2e → 2O -2 │3
Task No. 14
When aluminum oxide reacted with nitric acid, a salt was formed. The salt was dried and calcined. The solid residue formed during calcination was subjected to electrolysis in molten cryolite. The metal obtained by electrolysis was heated with a concentrated solution containing potassium nitrate and potassium hydroxide, and a gas with a pungent odor was released. Write equations for the four reactions described.
1) When amphoteric Al 2 O 3 interacts with nitric acid, a salt is formed - aluminum nitrate (exchange reaction):
Al 2 O 3 + 6HNO 3 → 2Al(NO 3) 3 + 3H 2 O
2) When aluminum nitrate is calcined, aluminum oxide is formed, and nitrogen dioxide and oxygen are also released (aluminum belongs to the group of metals (in the activity series from alkaline earth to Cu inclusive), the nitrates of which decompose to metal oxides, NO 2 and O 2):
3) Metallic aluminum is formed during the electrolysis of Al 2 O 3 in molten cryolite Na 2 AlF 6 at 960-970 o C.
Al 2 O 3 electrolysis scheme:
Dissociation of aluminum oxide occurs in the melt:
Al 2 O 3 → Al 3+ + AlO 3 3-
K(-): Al 3+ + 3e → Al 0
A(+): 4AlO 3 3- − 12e → 2Al 2 O 3 + 3O 2
Overall process equation:
Liquid aluminum collects at the bottom of the electrolyser.
4) When aluminum is treated with a concentrated alkaline solution containing potassium nitrate, ammonia is released and potassium tetrahydroxyaluminate is also formed (alkaline medium):
8Al + 5KOH + 3KNO 3 + 18H 2 O → 3NH 3 + 8K
Al 0 – 3e → Al +3 │8
N +5 + 8e → N -3 │3
Task No. 15
8AAA8C
Some iron(II) sulfide was divided into two parts. One of them was treated with hydrochloric acid, and the other was fired in air. When the released gases interacted, a simple yellow substance was formed. The resulting substance was heated with concentrated nitric acid, and a brown gas was released. Write equations for the four reactions described.
1) When iron (II) sulfide is treated with hydrochloric acid, iron (II) chloride is formed and hydrogen sulfide is released (exchange reaction):
FeS + 2HCl → FeCl 2 + H 2 S
2) When burning iron (II) sulfide, iron is oxidized to the oxidation state +3 (Fe 2 O 3 is formed) and sulfur dioxide is released:
3) When two sulfur-containing compounds SO 2 and H 2 S interact, an oxidation-reduction reaction (coproportionation) occurs, as a result of which sulfur is released:
2H 2 S + SO 2 → 3S↓ + 2H 2 O
S -2 – 2e → S 0 │2
S +4 + 4e → S 0 │1
4) When sulfur is heated with concentrated nitric acid, sulfuric acid and nitrogen dioxide are formed (redox reaction):
S + 6HNO 3 (conc.) → H 2 SO 4 + 6NO 2 + 2H 2 O
S 0 – 6e → S +6 │1
N +5 + e → N +4 │6
Task No. 16
The gas obtained by treating calcium nitride with water was passed over hot copper (II) oxide powder. The resulting solid was dissolved in concentrated nitric acid, the solution was evaporated, and the resulting solid residue was calcined. Write down equations for the four reactions described.
1) Calcium nitride reacts with water, forming alkali and ammonia:
Ca 3 N 2 + 6H 2 O → 3Ca(OH) 2 + 2NH 3
2) By passing ammonia over a hot powder of copper (II) oxide, the copper in the oxide is reduced to metallic, and nitrogen is released (hydrogen, coal, carbon monoxide, etc. are also used as reducing agents):
Cu +2 + 2e → Cu 0 │3
2N -3 – 6e → N 2 0 │1
3) Copper, located in the series of metal activities after hydrogen, reacts with concentrated nitric acid to form copper nitrate and nitrogen dioxide:
Cu + 4HNO 3(conc.) → Cu(NO 3) 2 + 2NO 2 + 2H 2 O
Cu 0 - 2e → Cu +2 │1
N +5 +e → N +4 │2
4) When copper nitrate is calcined, copper oxide is formed, and nitrogen dioxide and oxygen are also released (copper belongs to the group of metals (in the activity series from alkaline earth to Cu inclusive), the nitrates of which decompose to metal oxides, NO 2 and O 2):
Task No. 17
Silicon was burned in a chlorine atmosphere. The resulting chloride was treated with water. The precipitate released was calcined. Then fused with calcium phosphate and coal. Write down equations for the four reactions described.
1) The reaction between silicon and chlorine occurs at a temperature of 340-420 o C in a flow of argon with the formation of silicon (IV) chloride:
2) Silicon (IV) chloride is completely hydrolyzed, resulting in the formation of hydrochloric acid, and silicic acid precipitates:
SiCl 4 + 3H 2 O → H 2 SiO 3 ↓ + 4HCl
3) When calcined, silicic acid decomposes to silicon (IV) oxide and water:
4) When silicon dioxide is fused with coal and calcium phosphate, an oxidation-reduction reaction occurs, resulting in the formation of calcium silicate, phosphorus, and the release of carbon monoxide:
C 0 − 2e → C +2 │10
4P +5 +20e → P 4 0 │1
Task No. 18
Note! This format of tasks is outdated, but nevertheless tasks of this type deserve attention, since in fact they require writing down the same equations that are found in the Unified State Exam KIMs of the new format.
The following substances are given: iron, iron scale, dilute hydrochloric and concentrated nitric acid. Write equations for four possible reactions between all the proposed substances, without repeating pairs of reactants.
1) Hydrochloric acid reacts with iron, oxidizing it to the oxidation state +2, and hydrogen is released (substitution reaction):
Fe + 2HCl → FeCl 2 + H 2
2) Concentrated nitric acid passivates iron (i.e., a strong protective oxide film is formed on its surface), however, under the influence of high temperature, iron is oxidized by concentrated nitric acid to oxidation state +3:
3) The formula of iron scale is Fe 3 O 4 (a mixture of iron oxides FeO and Fe 2 O 3). Fe 3 O 4 enters into an exchange reaction with hydrochloric acid, resulting in a mixture of two iron (II) and (III) chlorides:
Fe 3 O 4 + 8HCl → 2FeCl 3 + FeCl 2 + 4H 2 O
4) In addition, iron scale enters into a redox reaction with concentrated nitric acid, and the Fe +2 contained in it is oxidized to Fe +3:
Fe 3 O 4 + 10HNO 3 (conc.) → 3Fe(NO 3) 3 + NO 2 + 5H 2 O
5) Iron scale and iron, when sintered, enter into a comporportionation reaction (the same chemical element acts as an oxidizing agent and a reducing agent):
Task No. 19
The following substances are given: phosphorus, chlorine, aqueous solutions of sulfuric acid and potassium hydroxide. Write equations for four possible reactions between all the proposed substances, without repeating pairs of reactants.
1) Chlorine is a poisonous gas with high chemical activity and reacts especially vigorously with red phosphorus. In an atmosphere of chlorine, phosphorus spontaneously ignites and burns with a weak greenish flame. Depending on the ratio of the reactants, phosphorus (III) chloride or phosphorus (V) chloride can be obtained:
2P (red) + 3Cl 2 → 2PCl 3
2P (red) + 5Cl 2 → 2PCl 5
Cl 2 + 2KOH → KCl + KClO + H 2 O
If chlorine is passed through a hot concentrated alkali solution, the molecular chlorine is disproportionated into Cl +5 and Cl -1, resulting in the formation of chlorate and chloride, respectively:
3) As a result of the interaction of aqueous solutions of alkali and sulfuric acid, an acidic or average salt of sulfuric acid is formed (depending on the concentration of the reagents):
KOH + H2SO4 → KHSO4 + H2O
2KOH + H 2 SO 4 → K 2 SO 4 + 2H 2 O (neutralization reaction)
4) Strong oxidizing agents such as sulfuric acid convert phosphorus into phosphoric acid:
2P + 5H 2 SO 4 → 2H 3 PO 4 + 5SO 2 + 2H 2 O
Task No. 20
The substances given are: nitric oxide (IV), copper, potassium hydroxide solution and concentrated sulfuric acid. Write equations for four possible reactions between all the proposed substances, without repeating pairs of reactants.
1) Copper, located in the series of metal activities to the right of hydrogen, is capable of oxidation by strong oxidizing acids (H 2 SO 4 (conc.), HNO 3, etc.):
Cu + 2H 2 SO 4 (conc.) → CuSO 4 + SO 2 + 2H 2 O
2) As a result of the interaction of a KOH solution with concentrated sulfuric acid, an acid salt is formed - potassium hydrogen sulfate:
KOH + H 2 SO 4 (conc.) → KHSO 4 + H 2 O
3) When passing brown gas, NO 2 N +4 is disproportioned into N +5 and N +3, resulting in the formation of potassium nitrate and nitrite, respectively:
2NO 2 + 2KOH → KNO 3 + KNO 2 + H 2 O
4) When brown gas is passed through a concentrated solution of sulfuric acid, N +4 is oxidized to N +5 and sulfur dioxide is released:
2NO 2 + H 2 SO 4 (conc.) → 2HNO 3 + SO 2
Task No. 21
The following substances are given: chlorine, sodium hydrosulfide, potassium hydroxide (solution), iron. Write equations for four possible reactions between all the proposed substances, without repeating pairs of reactants.
1) Chlorine, being a strong oxidizing agent, reacts with iron, oxidizing it to Fe +3:
2Fe + 3Cl 2 → 2FeCl 3
2) When chlorine is passed through a cold concentrated alkali solution, chloride and hypochlorite are formed (molecular chlorine is disproportionate to Cl +1 and Cl -1):
2KOH + Cl 2 → KCl + KClO + H 2 O
If chlorine is passed through a hot concentrated alkali solution, the molecular chlorine disproportionates into Cl +5 and Cl -1, resulting in the formation of chlorate and chloride, respectively:
3Cl 2 + 6KOH → 5KCl + KClO 3 + 3H 2 O
3) Chlorine, which has stronger oxidizing properties, is capable of oxidizing the sulfur contained in the acid salt:
Cl 2 + NaHS → NaCl + HCl + S↓
4) Acid salt - sodium hydrosulfide in an alkaline environment turns into sulfide:
2NaHS + 2KOH → K 2 S + Na 2 S + 2H 2 O
Iron(II) salts
2FeSO 4 + Br 2 + H 2 SO 4 = Fe 2 (SO 4) 3 + 2HBr
FeBr 2 + 4HNO 3 (conc.) = Fe(NO 3) 3 + 2HBr + NO 2 + H 2 O
6FeSO 4 + K 2 Cr 2 O 7 + 7H 2 SO 4 = 3Fe 2 (SO 4) 3 + Cr 2 (SO 4) 3 + K 2 SO 4 + 7H 2 O
2FeSO 4 + H 2 O 2 + H 2 SO 4 = Fe 2 (SO 4) 3 + 2H 2 O
6FeSO 4 + 2KNO 3 + 4H 2 SO 4 = 3Fe 2 (SO 4) 3 + 2NO + K 2 SO 4 + 4H 2 O
2FeSO 4 + CuSO 4 = Fe 2 (SO 4) 3 + Cu
4FeSO 4 + O 2 + 2H 2 SO 4 = 2Fe 2 (SO 4) 3 + 2H 2 O
10FeSO 4 + 2KMnO 4 + 8H 2 SO 4 = 5Fe 2 (SO 4) 3 + 2MnSO 4 + K 2 SO 4 + 8H 2 O
FeCl 2 + NaNO 2 + 2HCl = FeCl 3 + NO + NaCl + H 2 O
5FeCl 2 + 3KMnO 4 + 24HCl = 5FeCl 3 + 3MnCl 2 + 5Cl 2 + 3KCl + 12H 2 O
2FeCl 2 + 2H 2 O ![]() Fe + H 2 + 2Cl 2 + Fe(OH) 2 ↓
Fe + H 2 + 2Cl 2 + Fe(OH) 2 ↓
4FeCl 2 + 8NaOH + O 2 + 2H 2 O = 4Fe(OH) 3 ↓ + 8NaCl
2FeCl 2 + Cl 2 + 6NaOH = Fe(OH) 3 + 6NaCl + 3H 2 O
FeCl 2 + 4HNO 3 (conc.) = Fe(NO 3) 3 + NO 2 + 2HCl + H 2 O
3FeCl 2 + 4HNO 3 = Fe(NO 3) 3 + 2FeCl 3 + NO + 2H 2 O
2FeCl 2 + Cl 2 = 2FeCl 3
2FeI 2 + 6H 2 SO 4 (conc.) = Fe 2 (SO 4) 3 + 2I 2 + 3SO 2 + 6H 2 O
2FeS + 7Cl 2 = 2FeCl 3 + 2SCl 4
10FeS + 6KMnO 4 + 24H 2 SO 4 = 5Fe 2 (SO 4) 3 + 6MnSO 4 + 3K 2 SO 4 + 24H 2 O
4FeS + 7O 2 = 2Fe 2 O 3 + 4SO 2
FeS + 12HNO3(conc.) = Fe(NO3)3 + H2SO4 + 9NO2 + 5H2O
Saltsgland(III)
FeCl 3 + 3NaOH = Fe(OH) 3 ↓ + 3NaCl
2FeCl 3 + 3Na 2 S = 2FeS + S + 6NaCl
2FeCl 3 + Fe = 3FeCl 2
2FeCl 3 + 2KI = 2FeCl 2 + I 2 + 2KCl
2FeCl 3 + H 2 S = 2FeCl 2 + S + 2HCl
2FeCl 3 + Cu = 2FeCl 2 + CuCl 2
2FeCl 3 + H 2 = 2FeCl 2 + 2HCl
FeCl 3 + 3CH 3 COOAg = (CH 3 COO) 3 Fe + 3AgCl↓
4FeCl 3 + 6H 2 O ![]() 2Fe + 3H 2 + 2Fe(OH) 3 + 6Cl 2
2Fe + 3H 2 + 2Fe(OH) 3 + 6Cl 2
2FeBr 3 + K 2 S + 4KOH = 2Fe(OH) 2 ↓ + S↓ + 6KBr
2Fe(NO 3) 3 + 3Zn = 2Fe + 3Zn(NO 2) 2
2Fe(NO 3) 3 + 4H 2 SO 4 (conc.) = Fe 2 (SO 4) 3 + SO 2 + 4HNO 3 + 2H 2 O
Fe(NO 3) 2 + Na 2 S = FeS↓ + 2NaNO 3
Fe 2 (SO 4) 3 + 2KI = I 2 + 2FeSO 4 + K 2 SO 4
Fe 2 (SO 4) 3 + 3BaI 2 = 2FeI 2 + 3BaSO 4 ↓ + I 2 ↓
Fe 2 (SO 4) 3 + 3BaCl 2 = 3BaSO 4 ↓ + 2FeCl 3
2NaFeO 2 + 3NaNO 3 + 2NaOH 2Na 2 FeO 4 + 3NaNO 2 + H 2 O
2K 2 FeO 4 + 16HCl = 4KCl + 2FeCl 3 + 3Cl 2 + 8H 2 O
4Fe(NO 3) 3 2Fe 2 O 3 + 12NO 2 + 3O 2
When mixing solutions, hydrolysis occurs at both the weak base cation and the weak acid anion:
2Fe(NO 3) 3 + 3K 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6KNO 3
2FeCl 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6NaCl
2FeCl 3 + 3Na 2 SO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3SO 2 + 6NaCl
2Fe(NO 3) 3 + 3Na 2 CO 3 + H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6NaNO 3
Fe 2 (SO 4) 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 3Na 2 SO 4
Fe 2 (SO 4) 3 + Na 2 SO 3 + H 2 O = Na 2 SO 4 + 2FeSO 4 + H 2 SO 4
Iron. Iron compounds.
1. The salt obtained by dissolving iron in concentrated sulfuric acid was treated with an excess of sodium hydroxide solution. The brown precipitate that formed was filtered and calcined. The resulting substance was fused with iron. Write the equations for the reactions described.
2. The precipitate obtained from the reaction of iron (III) chloride and silver nitrate was filtered. The filtrate was treated with a solution of potassium hydroxide. The brown precipitate that formed was separated and calcined. When heated, the resulting substance reacts with aluminum, releasing heat and light. Write the equations for the reactions described.
3. The gas released when hydrogen chloride reacts with potassium permanganate reacts with iron. The reaction product was dissolved in water and sodium sulfide was added to it. The lighter of the resulting insoluble substances was separated and reacted with hot concentrated nitric acid. Write the equations for the reactions described.
4. The foul-smelling liquid formed by the reaction of hydrogen bromide with potassium permanganate was separated and heated with iron filings. The reaction product was dissolved in water and a solution of cesium hydroxide was added to it. The resulting precipitate was filtered and heated. Write the equations for the reactions described.
5. The substance obtained at the cathode by electrolysis of a solution of iron (II) chloride was fused with sulfur, and the product of this reaction was calcined. The resulting gas was passed through a solution of barium hydroxide. Write the equations for the reactions described.
6. A solution of iron(III) chloride was electrolyzed with graphite electrodes. The brown precipitate formed as a by-product of electrolysis was filtered and calcined. The substance formed at the cathode was dissolved in concentrated nitric acid when heated. The product released at the anode was passed through a cold solution of potassium hydroxide. Write the equations for the reactions described.
7. The solution of ferric chloride was treated with a solution of sodium hydroxide, the precipitate that formed was separated and heated. The solid reaction product was mixed with soda ash and calcined. Sodium nitrate and hydroxide were added to the remaining substance and heated at high temperature for a long time. Write the equations for the reactions described.
8. Ferrous oxide was heated with dilute nitric acid. The solution was carefully evaporated, the solid residue was dissolved in water, iron powder was added to the resulting solution and after some time filtered. A solution of potassium hydroxide was added to the filtrate, the precipitate that formed was separated and left in air, and the color of the substance changed. Write the equations for the reactions described.
9. Ferric chloride was treated with concentrated nitric acid while heating and the solution was carefully evaporated. The solid product was dissolved in water, potash was added to the resulting solution, and the precipitate that formed was separated and calcined. Hydrogen gas was passed over the resulting substance while heating. Write the equations for the reactions described.
10. Soda ash was added to the ferric chloride solution and the precipitate that formed was separated and calcined. Carbon monoxide was passed over the resulting substance while heating, and the solid product of the last reaction was introduced into interaction with bromine. Write the equations for the reactions described.
11. Iron scale was dissolved in concentrated nitric acid while heating. The solution was carefully evaporated and the reaction product was dissolved in water. Iron powder was added to the resulting solution, after some time the solution was filtered, and the filtrate was treated with a solution of potassium hydroxide, resulting in the formation of a light green precipitate, which quickly darkens in air. Write the equations for the reactions described.
12. Iron powder was added to the ferric chloride solution and after some time the solution was filtered. Sodium hydroxide was added to the filtrate, the resulting precipitate was separated and treated with hydrogen peroxide. An excess of caustic potassium solution and bromine were added to the resulting substance; As a result of the reaction, the color of bromine disappeared. Write the equations for the reactions described.
13. The insoluble substance formed when sodium hydroxide is added to a solution of ferric chloride was separated and dissolved in dilute sulfuric acid. Zinc dust was added to the resulting solution, the resulting precipitate was filtered and dissolved in concentrated hydrochloric acid. Write the equations for the reactions described.
14. Iron powder was dissolved in a large amount of dilute sulfuric acid and air was passed through the resulting solution, and then a gas with the smell of rotten eggs was passed through. The resulting insoluble salt was separated and dissolved in a hot solution of concentrated nitric acid. Write the equations for the reactions described.
15. Unknown substance A dissolves in concentrated hydrochloric acid, the dissolution process is accompanied by the release of gas with the smell of rotten eggs; after neutralizing the solution with alkali, a voluminous white (light green) precipitate is formed. When substance A is fired, two oxides are formed. One of them is a gas that has a characteristic pungent odor and discolors bromine water with the formation of two strong acids in solution. Write the equations for the reactions described.
16. A silver-gray metal that is attracted by a magnet was added to hot concentrated sulfuric acid and heated. The solution was cooled and caustic soda was added until the formation of an amorphous brown precipitate ceased. The precipitate was separated, calcined and dissolved in concentrated hydrochloric acid while heating. Write the equations for the reactions described.
17. The substance obtained by heating iron scale in a hydrogen atmosphere was added to hot concentrated acid and heated. The resulting solution was evaporated, the residue was dissolved in water and treated with a solution of barium chloride. The solution was filtered and a copper plate was added to the filtrate, which dissolved after some time. Write the equations for the reactions described.
18. A solution of iron (III) chloride was subjected to electrolysis. The brown precipitate formed during electrolysis was filtered and dissolved in a solution of sodium hydroxide, after which the amount of sulfuric acid necessary to form a clear solution was added. The product released at the anode was passed through a hot solution of potassium hydroxide. Write the equations for the reactions described.
19. Iron was burned in chlorine. The reaction product was dissolved in water and iron filings were added to the solution. After some time, the solution was filtered and sodium sulfide was added to the filtrate. The resulting precipitate was separated and treated with 20% sulfuric acid, obtaining an almost colorless solution. Write the equations for the reactions described.
20. A mixture of iron powder and a solid product obtained by the reaction of sulfur dioxide and hydrogen sulfide was heated without access to air. The resulting product was fired in air. The resulting solid reacts with the aluminum, releasing large amounts of heat. Write the equations for the reactions described.
21. Iron (III) oxide was fused with soda. The resulting product was added to water. The precipitate that formed was dissolved in hydroiodic acid. The released halogen was bound with sodium thiosulfate. Write the equations for the reactions described.
22. Chlorine reacted with a hot solution of potassium hydroxide. As the solution cooled, crystals of Berthollet salt precipitated. The resulting crystals were added to a solution of hydrochloric acid. the resulting simple substance reacted with metallic iron. The reaction product was heated with a new portion of iron. Write the equations for the reactions described.
23. Pyrite was fired, and the resulting gas with a pungent odor was passed through hydrogen sulfide acid. The resulting yellowish precipitate was filtered, dried, mixed with concentrated nitric acid and heated. The resulting solution gives a precipitate containing barium nitrate. Write the equations for the reactions described.
24. Iron filings were dissolved in dilute sulfuric acid, the resulting solution was treated with an excess of sodium hydroxide solution. The resulting precipitate was filtered and left in air until it acquired a brown color. The brown substance was calcined to constant mass. Write the equations for the reactions described.
25. Conducted electrolysis of a sodium chloride solution. Iron (III) chloride was added to the resulting solution. The precipitate that formed was filtered and calcined. The solid residue was dissolved in hydroiodic acid. Write the equations for the reactions described.
26. Potassium chlorate was heated in the presence of a catalyst, and a colorless gas was released. By burning iron in an atmosphere of this gas, iron oxide was obtained. It was dissolved in dilute hydrochloric acid. To the resulting solution was added a solution containing sodium dichromate and hydrochloric acid. Write the equations for the reactions described.
27. Iron was burned in chlorine. The resulting salt was added to the sodium carbonate solution, and a brown precipitate formed. This precipitate was filtered and calcined. The resulting substance was dissolved in hydroiodic acid. Write the equations for the reactions described.
28. Sulfur was fused with iron. The reaction product was treated with hydrochloric acid. The gas released was burned in excess oxygen. The combustion products were absorbed by an aqueous solution of iron (III) sulfate. Write the equations for the reactions described.
29. As a result of incomplete combustion of coal, a gas was obtained, in a current of which iron (III) oxide was heated. The resulting substance was dissolved in hot concentrated sulfuric acid. The resulting salt solution was treated with an excess of potassium sulfide solution. Write the equations for the reactions described.
30. Iron was burned in a chlorine atmosphere. The resulting substance was treated with an excess of sodium hydroxide solution. The resulting brown precipitate was filtered and calcined. The residue after calcination was dissolved in hydroiodic acid. Write the equations for the reactions described.
31. Iron was dissolved in dilute nitric acid. An excess of sodium carbonate solution was added to the resulting solution. The precipitate that separated was filtered and calcined. The resulting substance was ground into a fine powder along with aluminum and the mixture was set on fire. It burned, releasing a large amount of heat. Write the equations for the reactions described.
32. Iron powder was heated with sulfur powder. The reaction product was dissolved in hydrochloric acid, and excess alkali was added to the solution. The precipitate that formed was calcined in a nitrogen atmosphere. Write the equations for the reactions described.
33. Iron was burned in a chlorine atmosphere. The resulting salt was dissolved in water and a solution of potassium iodide was added to it. The precipitate of a simple substance was separated and divided into two parts. The first was treated with dilute nitric acid, and the second was heated in a hydrogen atmosphere. Write the equations for the reactions described.
34. Iron was dissolved in hydrochloric acid, and sodium hydroxide was added to the resulting solution until the precipitation stopped. In the received
Solving the problems of part C2
1. A mixture of two colorless and odorless gases, A and B, was passed through when heated over a catalyst containing iron. The resulting gas B was passed into a solution of hydrobromic acid, and a neutralization reaction occurred. The solution was evaporated and the residue was heated with caustic potassium, resulting in the release of colorless gas B with a pungent odor. When gas B is burned in air, water and gas A are formed. Write the equation for the reactions described.
Solution
An acid solution can be neutralized with a substance that exhibits basic properties. Since when the reaction product with caustic potassium was heated, a gas with a pungent odor and a gas with basic properties were released, this gas is ammonia NH 3.
1 equation - synthesis of ammonia from nitrogen and hydrogen;
Equation 2 - acid neutralization;
3 equation - qualitative reaction to ammonia with alkali;
Equation 4 - combustion of ammonia in air, releasing nitrogen
Gases - N 2, H 2 and NH 3.
1) N 2 + 3H 2 ↔ 2NH 3
2) NH 3 + HBr = NH 4 Br
3) NH 4 Br + KOH = KBr + H 2 O + NH 3
4) 4NH 3 + 3O 2 = 2N 2 + 6 H 2 O
2. Let's pass sulfur dioxide through a solution of hydrogen peroxide. The water was evaporated and magnesium shavings were added to the residue. The released gas was passed through a solution of copper sulfate. The resulting black precipitate was separated and fired. Write the equation for the reactions described.
Solution
In sulfur dioxide, the oxidation state of sulfur is +4. Therefore, it can be both an oxidizing agent and a reducing agent. With a strong oxidizing agent, sulfur will be a reducing agent and will increase the oxidation number to +6 (i.e., H 2 SO 4 ) (1 equation).
After evaporation H 2 O, concentrated sulfuric acid is formed, which, interacting with Mg (active metal), produces hydrogen sulfide (2). Copper sulfate - II, reacting with hydrogen sulfide, will give copper sulfide - a black precipitate (3). When sulfides are roasted, sulfur oxide (IV) and metal oxide (4) are formed.
1) SO 2 + H 2 O 2 = H 2 SO 4
2) 5H 2 SO 4 conc. + 4Mg = 4MgSO 4 + H 2 S + 4H 2 O
3) H 2 S + CuSO 4 = CuS↓ + H 2 SO 4
4) 2CuS + 3O 2 = 2CuO + 2SO 2
3. When a certain mineral A, consisting of 2 elements, is fired, a gas is formed that has a pungent odor and discolors bromine water with the formation of two strong acids in solution. When substance B, consisting of the same elements as mineral A, but in a different ratio, interacts with concentrated hydrochloric acid, a gas with the smell of “rotten eggs” is released. When gases interact with each other, a simple yellow substance and water are formed. Write the equations for the reactions described.
Solution
Since when substance B is exposed to hydrochloric acid, hydrogen sulfide H is released 2 S (a gas with a “rotten egg” odor) (Equation 3), then both minerals are sulfides. Roasting of pyrite FeS is being studied in the sulfuric acid production process 2 (1). SO 2 – a gas with a pungent odor exhibits propertiesa reducing agent and reacting with bromine water produces two acids: sulfuric and hydrobromic (2). When sulfur dioxide (oxidizing agent) and hydrogen sulfide (reducing agent) interact, sulfur is formed - a simple yellow substance (4).
1) 4FeS 2 + 11O 2 = 2Fe 2 O 3 + 8SO 2
2) SO 2 + Br 2 + 2H 2 O = H 2 SO 4 + 2HBr
3) FeS + 2HCl = FeCl 2 + H 2 S
4) SO 2 + 2H 2 S = 3S↓ + 2H 2 O
4. Nitric acid was neutralized with baking soda, the solution was evaporated and the residue was calcined. The resulting substance was added to a solution of potassium permanganate acidified with sulfuric acid, and the solution became colorless. The nitrogen-containing reaction product was placed in a solution of caustic soda and zinc dust was added, and a gas with a sharp characteristic odor was released. Write the equations for the reactions described.
Solution
After neutralization of the solution, sodium nitrate is formed (1). Nitrates formed by metals in the voltage series to the left of Mg decompose to form nitrites and oxygen (2). Potassium permanganate KMnO 4 , which has a pink color, is a strong oxidizing agent in an acidic environment and oxidizes sodium to NaN nitrate+5 O 3 , itself is reduced to Mn+2 (colorless) (3). When zinc reacts with an alkali solution, atomic hydrogen is released, which is a very strong reducing agent, therefore sodium nitrate NaN+5 O 3 is reduced to ammonia N-3 H 3 (4).
1) HNO 3 + NaHCO 3 = NaNO 3 + H 2 O + CO 2
2) 2 NaNO 3 = 2NaNO 2 + O 2
3) 5NaNO 2 + 2KMnO 4 + 3H 2 SO 4 = 5NaNO 3 + K 2 SO 4 + 2MnSO 4 + 3H 2 O
4) NaNO 3 + 4Zn+ 7NaOH + 6H 2 O = NH 3 + 4Na 2 Zn(OH) 4
5. An unknown metal was burned in oxygen. The reaction product, interacting with carbon dioxide, forms two substances: a solid that reacts with a solution of hydrochloric acid to release carbon dioxide, and a gaseous simple substance that supports combustion. Write the equations for the reactions described.
Solution
The gas that supports combustion is oxygen (4). When metals burn in oxygen, oxides and peroxides can form. Oxides will give only one substance when interacting with carbon dioxide - a carbonate salt, so we take an alkali metal, sodium, which forms peroxide (1). When reacting with carbon dioxide, salt is formed and oxygen is released (2). Carbonate with acid produces carbon dioxide (3).
1) 2Na + O 2 = Na 2 O 2
2) 2Na 2 O 2 + 2CO 2 = 2Na 2 CO 3 + O 2
3) Na 2 CO 3 + 2HCl = 2NaCl + H 2 O + CO 2
4) O 2 +C = CO 2.
6. Trivalent chromium hydroxide was treated with hydrochloric acid. Potash was added to the resulting solution, the precipitate that formed was separated and added to a concentrated solution of potassium hydroxide, as a result of which the precipitate dissolved. After adding excess hydrochloric acid, a green solution was obtained. Write the equations for the reactions described.
Solution
Chromium hydroxide Cr(OH) 3 - amphoteric. With hydrochloric acid will give CrCl 3 (1), the salt is formed by a weak base and a strong acid, so it will undergo cationic hydrolysis. Potash - potassium carbonate K 2 CO 3 formed by a strong base and a weak acid, undergoes hydrolysis at the anion. The two salts mutually enhance the hydrolysis of each other, so the hydrolysis proceeds to the end: until the formation of Cr(OH) 3 and CO 2 (2). Cr(OH)3 in excess of alkali gives potassium hexahydroxochromite K 3 Cr(OH) 6 (3). When exposed to an excess of strong acid, two salts are formed (4).
1) Cr(OH) 3 + 3HCl = CrCl 3 + 3H 2 O
2) CrCl 3 + 3K 2 CO 3 + 3H 2 O = 2Cr(OH) 3 ↓ + 3CO 2 + 6KCl
3) Cr(OH) 3 + 3KOH conc. = K 3 Cr(OH) 6
4) K 3 Cr(OH) 6 + 6HCl = CrCl 3 + 3KCl + 6H 2 O.
7. The product of the reaction of lithium with hydrogen was treated with water. The released gas was mixed with excess oxygen and passed over a platinum catalyst while heating; the resulting gas mixture was brown in color. Write the equations for the reactions described.
Solution
The interaction of nitrogen and lithium produces lithium nitride (1), which decomposes with water to release ammonia (2). Ammonia is oxidized by oxygen in the presence of a platinum catalyst to nitrogen oxide (II), which has no color (3). Formation of brown gas NO 2 from NO occurs spontaneously (4).
1) 6Li + N 2 = 2Li 3 N
2) Li 3 N + 3H 2 O = 3LiOH + NH 3
3) 4NH 3 + 5O 2 = 4NO + 6H 2 O
4) 2NO + O 2 = 2NO 2.
8. Magnesium silicide was treated with a solution of hydrochloric acid and the resulting gas was burned. The solid reaction product was mixed with soda ash, the mixture was heated until melting and kept for some time. After cooling, the reaction product (used under the name “liquid glass”) was dissolved in water and treated with a solution of sulfuric acid. Write equations for descriptive reactions.
Solution
When magnesium silicide reacts with hydrochloric acid, silane gas (1) is formed. It ignites spontaneously in air, producing silica (solid) and water (2). When silicon oxide is fused with alkali or soda, sodium silicate (“liquid glass”) is formed (3). Sulfuric acid, being stronger, displaces weak silicic acid from solution, which is insoluble in water (4).
1) Mg 2 Si + 4HCl = 2MgCl 2 + SiH 4
2) 2SiH 4 + 2O 2 = SiO 2 + 2H 2 O
3) SiO 2 + Na 2 CO 3 = Na 2 SiO 3 + CO 2
4) Na 2 SiO 3 + H 2 SO 4 = Na 2 SO 4 + H 2 SiO 3 ↓.
9. When the orange substance is heated, it decomposes; decomposition products include a colorless gas and a green solid. The released gas reacts with lithium even with slight heating. The product of the latter reaction reacts with water, releasing a gas with a pungent odor that can reduce metals, such as copper, from their oxides. Write the equations for the reactions described.
Solution
A gas with a pungent odor that can reduce metals from their oxides (equation 4) is ammonia (equation 3). An orange substance that decomposes to release nitrogen (a colorless gas) and form a green solid, Cr. 2 O 3 - ammonium dichromate (NH 4) 2 Cr 2 O 7 (equation 1), when lithium nitride reacts with water, ammonia is released (3).
1) (NH 4 ) 2 Cr 2 O 7 = t N 2 + 4H 2 O + Cr 2 O 3
2) N 2 + 6Li = 2Li 3 N
3) Li 3 N + 3H 2 O = 3LiOH + NH 3
4) 2NH 3 + 3CuO = N 2 + 3Cu + 3H 2 O.
10. An unknown red substance was heated in chlorine and the reaction product was dissolved in water. Alkalies were added to the resulting solution, the resulting blue precipitate was filtered and calcined. When heating the calcination product, which is black, with coke, a red starting material was obtained. Write the equations for the reactions described.
Solution
Red metal - copper. When heated with chlorine, copper-II chloride CuCl is formed 2 (1). When alkali is added to a solution, a gelatinous blue precipitate Cu(OH) precipitates. 2 - copper-II hydroxide (2). When heated, it decomposes into black copper-II oxide (3). When the oxide is heated with coke (C), copper is reduced.
1) Cu + Cl 2 = CuCl 2
2) CuCl 2 + 2NaOH = Cu(OH) 2 ↓ + 2NaCl
3) Cu(OH) 2 = CuO + H 2 O
4) CuO + C = Cu + CO.
11. The salt obtained by reacting zinc oxide with sulfuric acid was calcined at 800 O C. The solid reaction product was treated with a concentrated alkali solution and carbon dioxide was passed through the resulting solution. Write the reaction equations for the transformations described.
Solution
When zinc oxide reacts with sulfuric acid, the salt zinc sulfate ZnSO is obtained 4 (1). At high temperatures, many metal sulfates decompose to form metal oxide, sulfur dioxide, and oxygen (2). Zinc oxide is amphoteric, therefore it reacts with alkali, forming sodium tetrahydroxyzincate Na 2 Zn(OH) 4 (3). When carbon dioxide is passed into water, carbonic acid is formed, which destroys the complex, and a precipitate of zinc hydroxide is formed (4).
1) ZnO + H 2 SO 4 = ZnSO 4 + H 2 O
2) 2ZnSO 4 = 2ZnO + SO 2 + O 2
3) ZnO + 2NaOH + H 2 O = Na 2 Zn(OH) 4
4) Na 2 Zn(OH) 4 + CO 2 = Na 2 CO 3 + Zn(OH) 2 ↓ + H 2 O.
12. Copper shavings were added to a solution of mercury-II nitrate. The solution was filtered and the filtrate was added dropwise to a solution containing sodium hydroxide and ammonium hydroxide. In this case, a short-term formation of a precipitate was observed, which dissolved to form a bright blue solution. When an excess of sulfuric acid solution was added to the resulting solution, a color change occurred. Write the equation for the reactions described.
Solution
Copper is in the series of metal stresses to the left of mercury, therefore it displaces it from the salt solution (1). When a solution of copper-II nitrate is added to alkali, insoluble copper-II hydroxide Cu(OH) is formed 2 (2), which dissolves in excess ammonia, forming a bright blue complex compound Cu(NH 3 ) 4 (OH) 2 (3). When sulfuric acid is added, it is destroyed and the solution turns blue (4).
1) Hg(NO 3 ) 2 + Cu = Ng + Cu(NO 3 ) 2
2) Cu(NO 3 ) 2 + 2KOH = Cu(OH) 2 ↓ + 2KNO 3
3) Cu(OH) 2 + 4NH 4 OH = Cu(NH 3 ) 4 (OH) 2 + 4H 2 O
4) Cu(NH 3 ) 4 (OH) 2 + 5H 2 SO 4 = CuSO 4 + 4NH 4 HSO 4 + 2H 2 O
acidic salt is formed, because excess acid.
13. Red phosphorus was burned in an atmosphere of chlorine and a few drops of water were added to the reaction product. The released substance was dissolved in excess water, iron powder was added to the resulting solution, and the gaseous reaction product was passed over a heated copper plate oxidized to cuprous oxide. Write the reaction equations for the transformations described.
Solution
When phosphorus burns in excess chlorine, phosphorus chloride-V PCl is formed 5 (1). Upon hydrolysis with a small amount of water, hydrogen chloride is released and metaphosphoric acid is formed (2). Iron displaces hydrogen from acid solutions (3). Hydrogen reduces metals from their oxides (4).
1) 2P + 5Cl 2 = 2PCl 5
2) PCl 5 + 3H 2 O = HPO 3 + 5HCl
3) Fe + 2HCl = FeCl 2 + H 2
4) CuO + H 2 = t Cu + H 2 O.
14. The substance obtained by heating iron scale in a hydrogen atmosphere was added to hot concentrated sulfuric acid and heated. The resulting solution was evaporated, the residue was dissolved in water and treated with a solution of barium chloride. The solution was filtered and a copper plate was added to the filtrate, which dissolved after some time. Write the equations for the reactions described.
Solution
When heating metal oxides, in particular iron scale Fe 3 O 4, with hydrogen metals are reduced (1). Iron does not react with concentrated sulfuric acid under normal conditions, but when heated it dissolves (2). Iron-III sulfate with barium chloride forms a precipitate of barium sulfate (30). Iron-III chloride exhibits oxidizing properties and dissolves copper (4).
1) Fe 3 O 4 + 8H 2 = 3Fe + 4H 2 O
2) 2Fe + 6H 2 SO 4conc. (hor.) = Fe 2 (SO 4 ) 3 + 3SO 2 + 6H 2 O
3) Fe 2 (SO 4 ) 3 + 3BaCl 2 = 3BaSO 4 ↓ + 2FeCl 3
4) 2FeCl 3 + Cu = 2FeCl 2 + CuCl 2.
15. Quicklime was calcined with excess coke. The reaction product after treatment with water is used to absorb sulfur dioxide and carbon dioxide. Write the equations for the reactions described.
Solution
Calcination of quicklime with coke is an industrial method for producing calcium carbide (1). When calcium carbide is hydrolyzed, acetylene is released and calcium hydroxide is formed (2), which can react with acid oxides (3, 4).
1) CaO + 3C = CaC 2 + CO
2) CaC 2 + 2H 2 O = Ca(OH) 2 ↓ + C 2 H 2
3) Ca(OH) 2 + SO 2 = CaSO 3 ↓ + H 2 O
4) Ca(OH) 2 + CO 2 = CaCO 3 ↓ + H 2 O.
16. Electrical discharges were passed over the surface of a caustic soda solution poured into a flask, and the air in the flask turned brown, which disappeared after some time. The resulting solution was carefully evaporated and it was determined that the solid residue was a mixture of two salts. When this mixture is heated, gas is released and the only substance remains. Write the equations for the reactions described.
Solution
During electrical discharges, nitrogen reacts with oxygen to form a colorless gas of nitric oxide (1), which is spontaneously quickly oxidized by atmospheric oxygen to brown nitric oxide-IV (2). Nitric oxide-IV, dissolving in alkali, forms two salts - nitrate and nitrite, because is an anhydride of two acids (3). When heated, nitrate decomposes to form nitrite and release oxygen (4).
1) N 2 + O 2 = 2NO
2) 2NO + O 2 = 2NO 2
3) 2NO 2 + 2NaOH = NaNO 3 + NaNO 2 + H 2 O
4) 2NaNO 3 = 2NaNO 2 + O 2.
17. A solution of hydrochloric acid was carefully added to the pyrolusite. The released gas was passed into a beaker half filled with a cold solution of potassium hydroxide. After the reaction was completed, the glass was covered with cardboard and left in the light; after a while they brought in a smoldering splinter, which flared up brightly. Write the equations for the reactions described.
Solution
Interaction of hydrochloric acid with pyrolusite MnO 2 - laboratory method for producing chlorine (1). Chlorine in a cold solution of potassium hydroxide gives two salts: potassium chloride and potassium hypochlorite (2). Hypochlorite is an unstable substance and, when illuminated, decomposes with the release of oxygen (3), the formation of which is proven with the help of a flashing splinter (4).
1) MnO 2 + 4HCl = Cl 2 + MnCl 2 + 2H 2 O
2) Cl 2 + 2KOH = KCl + KClO + H 2 O
3) 2KClO = 2KCl + O 2
4) C + O 2 = CO 2.
TASK for 04/10/19
1. To a 20% salt solution obtained by dissolving 12.5 g of copper sulfate (CuSO 4 5H 2 O) in water, 5.6 g of iron was added. After the reaction was completed, 117 g of a 10% sodium sulfide solution was added to the solution. Determine the mass fraction of sodium sulfide in the final solution (neglect hydrolysis processes). In your answer, write down the reaction equations that are indicated in the problem statement and provide all the necessary calculations (indicate the units of measurement of the original physical quantities).
2. Powder obtained by sintering 2.16 g of aluminum and 6.4 g of iron (III) oxide was added to a 20% salt solution obtained by dissolving 25 g of copper (II) sulfate pentahydrate in water. Determine the mass fraction of copper (II) sulfate in the resulting solution (neglect hydrolysis processes). In your answer, write down the reaction equations that are indicated in the problem statement and provide all the necessary calculations (indicate the units of measurement of the original physical quantities).
3. 18.2 g of calcium phosphide were added to 182.5 g of a 20% hydrochloric acid solution. Next, 200.2 g of Na 2 CO 3 · 10H 2 O was added to the resulting solution. Determine the mass fraction of sodium carbonate in the resulting solution (neglect hydrolysis processes). In your answer, write down the reaction equations that are indicated in the problem statement and provide all the necessary calculations (indicate the units of measurement of the original physical quantities).
Task for 02.05
1. Metallic zinc was added to concentrated sulfuric acid. The resulting salt was isolated, dissolved in water, and barium nitrate was added to the solution. After separating the precipitate, magnesium shavings were added to the solution, the solution was filtered, the filtrate was evaporated and calcined. Write the equations for the reactions described.
2. Iron scale was dissolved in concentrated nitric acid when heated. The solution was carefully evaporated and the reaction product was dissolved in water. Iron powder was added to the resulting solution, after some time the solution was filtered and the filtrate was treated with a solution of caustic potassium, resulting in a light green precipitate that quickly darkened in air. Write the equations for the reactions described.
3. Chromium (III) sulfide was treated with water, gas was released and an insoluble substance remained. A solution of caustic soda was added to this substance and chlorine gas was passed through, and the solution acquired a yellow color. The solution was acidified with sulfuric acid, as a result the color changed to orange; The gas released when the sulfide was treated with water was passed through the resulting solution, and the color of the solution changed to green. Write the equations for the reactions described.
Assignment for 04/29.
1. The solution of ferric chloride was treated with a solution of sodium hydroxide, the precipitate that formed was separated and heated. The solid reaction product was mixed with soda ash and calcined. Sodium nitrate and hydroxide were added to the remaining substance and heated for a long time at high temperature. Write the equations for the reactions described.
2. Ferrous oxide was heated with dilute nitric acid. The solution was carefully evaporated, the solid residue was dissolved in water, iron powder was added to the resulting solution and filtered after some time. A solution of potassium hydroxide was added to the filtrate, the precipitate that formed was separated and left in air, and the color of the substance changed. Write the equations for the reactions described.
3. Ferric chloride was treated with concentrated nitric acid while heating and the solution was carefully evaporated. The solid product was dissolved in water, potash was added to the resulting solution, and the precipitate that formed was separated and calcined. Hydrogen gas was passed over the resulting substance while heating. Write the equations for the reactions described.
TASKS for 24.04
1. Soda ash was added to the solution of trivalent chromium sulfate. The resulting precipitate was separated, transferred to a solution of sodium hydroxide, bromine was added and heated. After neutralizing the reaction products with sulfuric acid, the solution acquires an orange color, which disappears after passing sulfur dioxide gas through the solution. Write the equations for the reactions described.
2. Metallic zinc was added to concentrated sulfuric acid. The resulting salt was isolated, dissolved in water, and barium nitrate was added to the solution. After separating the precipitate, magnesium shavings were added to the solution, the solution was filtered, the filtrate was evaporated and calcined. Write the equations for the reactions described.
3. Zinc nitrate was calcined, and the reaction product was treated with sodium hydroxide solution when heated. Carbon dioxide was passed through the resulting solution until the precipitation ceased, after which it was treated with an excess of concentrated ammonia, and the precipitate dissolved. Write the equations for the reactions described.
4. Copper (I) oxide was treated with concentrated nitric acid, the solution was carefully evaporated and the solid residue was calcined. The gaseous reaction products were passed through a large amount of water and magnesium shavings were added to the resulting solution, resulting in the release of a gas used in medicine. Write the equations for the reactions described.
5. The solid formed when malachite is heated was heated in a hydrogen atmosphere. The reaction product was treated with concentrated sulfuric acid and, after separation from the sulfuric acid, added to a sodium chloride solution containing copper filings, resulting in the formation of a precipitate. Write the equations for the reactions described.
===========================================================================
Task 18.03
1. A zinc plate weighing 80 g is immersed in a solution containing 30 g of copper (II) chloride. Its mass has changed by 0.2 g. Determine whether all the salt has reacted. If not, what is its mass in solution?
2. To the solution obtained by adding 4 g of potassium hydride to 100 g of water, 100 ml of a 39% solution of nitric acid (density 1.24 g/ml) was added. Determine the mass fractions of all substances in the final solution
C2. 1. The solution obtained by passing sulfur dioxide through bromine water was neutralized with barium hydroxide. The precipitate that formed was separated, mixed with coke and calcined. When the calcination product is treated with hydrochloric acid, a gas is released with the smell of rotten eggs. Write the equations for the reactions described.
2. The substance formed by adding zinc powder to a solution of ferric chloride was separated by filtration and dissolved in hot dilute nitric acid. The solution was evaporated, the solid residue was calcined, and the released gases were passed through a solution of sodium hydroxide. Write the equations for the reactions described.
3. Sulfur dioxide is passedor through a solution of hydrogen peroxide. The water was evaporated from the resulting solution and magnesium shavings were added to the residue. The released gas was passed through a solution of copper sulfate. The black precipitate that fell out was separated and fired. Write the equations for the reactions described
4. A solution of hydrochloric acid was added to a water-insoluble white salt, which occurs in nature in the form of a mineral widely used in construction and architecture; as a result, the salt dissolved and a gas was released, which, when passed through lime water, produced a white precipitate. , which dissolved upon further passage of gas. When excess lime water is added to the resulting solution, a precipitate forms. Write the equations for the reactions described.
Option No. 1 on 31.01
Iron(III) salts
FeCl 3 + 3NaOH = Fe(OH) 3 ↓ + 3NaCl
2FeCl 3 + 3Na 2 S = 2FeS + S + 6NaCl
2FeCl 3 + Fe = 3FeCl 2
2FeCl 3 + H 2 S = 2FeCl 2 + S + 2HCl
2FeCl 3 + H 2 = 2FeCl 2 + 2HCl
FeCl 3 + 3CH 3 COOAg = (CH 3 COO) 3 Fe + 3AgCl↓
4FeCl 3 + 6H 2 O 2Fe + 3H 2 + 2Fe(OH) 3 + 6Cl 2
2Fe(NO 3) 3 + 3Zn = 2Fe + 3Zn(NO 2) 2
2Fe(NO 3) 3 + 4H 2 SO 4 (conc.) = Fe 2 (SO 4) 3 + SO 2 + 4HNO 3 + 2H 2 O
Fe(NO 3) 2 + Na 2 S = FeS↓ + 2NaNO 3
Fe 2 (SO 4) 3 + 2KI = I 2 + 2FeSO 4 + K 2 SO 4
Fe 2 (SO 4) 3 + 3BaI 2 = 2FeI 2 + 3BaSO 4 ↓ + I 2 ↓
Fe 2 (SO 4) 3 + 3BaCl 2 = 3BaSO 4 ↓ + 2FeCl 3
2K 2 FeO 4 + 16HCl = 4KCl + 2FeCl 3 + 3Cl 2 + 8H 2 O
4Fe(NO 3) 3 2Fe 2 O 3 + 12NO 2 + 3O 2
2FeCl 3 + 3Na 2 SO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3SO 2 + 6NaCl
2Fe(NO 3) 3 + 3Na 2 CO 3 + H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6NaNO 3
Fe 2 (SO 4) 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 3Na 2 SO 4
Fe 2 (SO 4) 3 + Na 2 SO 3 + H 2 O = Na 2 SO 4 + 2FeSO 4 + H 2 SO 4
Iron. Iron compounds.
1. The salt obtained by dissolving iron in concentrated sulfuric acid was treated with an excess of sodium hydroxide solution. The brown precipitate that formed was filtered and calcined. The resulting substance was fused with iron. Write the equations for the reactions described.
2. The precipitate obtained from the reaction of iron (III) chloride and silver nitrate was filtered. The filtrate was treated with a solution of potassium hydroxide. The brown precipitate that formed was separated and calcined. When heated, the resulting substance reacts with aluminum, releasing heat and light. Write the equations for the reactions described.
3. The gas released when hydrogen chloride reacts with potassium permanganate reacts with iron. The reaction product was dissolved in water and sodium sulfide was added to it. The lighter of the resulting insoluble substances was separated and reacted with hot concentrated nitric acid. Write the equations for the reactions described.
4. The foul-smelling liquid formed by the reaction of hydrogen bromide with potassium permanganate was separated and heated with iron filings. The reaction product was dissolved in water and a solution of cesium hydroxide was added to it. The resulting precipitate was filtered and heated. Write the equations for the reactions described.
5. The substance obtained at the cathode by electrolysis of a solution of iron (II) chloride was fused with sulfur, and the product of this reaction was calcined. The resulting gas was passed through a solution of barium hydroxide. Write the equations for the reactions described.
6. A solution of iron(III) chloride was electrolyzed with graphite electrodes. The brown precipitate formed as a by-product of electrolysis was filtered and calcined. The substance formed at the cathode was dissolved in concentrated nitric acid when heated. The product released at the anode was passed through a cold solution of potassium hydroxide. Write the equations for the reactions described.
7. The solution of ferric chloride was treated with a solution of sodium hydroxide, the precipitate that formed was separated and heated. The solid reaction product was mixed with soda ash and calcined. Sodium nitrate and hydroxide were added to the remaining substance and heated at high temperature for a long time. Write the equations for the reactions described.
8. Ferrous oxide was heated with dilute nitric acid. The solution was carefully evaporated, the solid residue was dissolved in water, iron powder was added to the resulting solution and after some time filtered. A solution of potassium hydroxide was added to the filtrate, the precipitate that formed was separated and left in air, and the color of the substance changed. Write the equations for the reactions described.
9. Ferric chloride was treated with concentrated nitric acid while heating and the solution was carefully evaporated. The solid product was dissolved in water, potash was added to the resulting solution, and the precipitate that formed was separated and calcined. Hydrogen gas was passed over the resulting substance while heating. Write the equations for the reactions described.
10. Soda ash was added to the ferric chloride solution and the precipitate that formed was separated and calcined. Carbon monoxide was passed over the resulting substance while heating, and the solid product of the last reaction was introduced into interaction with bromine. Write the equations for the reactions described.
11. Iron scale was dissolved in concentrated nitric acid while heating. The solution was carefully evaporated and the reaction product was dissolved in water. Iron powder was added to the resulting solution, after some time the solution was filtered, and the filtrate was treated with a solution of potassium hydroxide, resulting in the formation of a light green precipitate, which quickly darkens in air. Write the equations for the reactions described.
12. Iron powder was added to the ferric chloride solution and after some time the solution was filtered. Sodium hydroxide was added to the filtrate, the resulting precipitate was separated and treated with hydrogen peroxide. An excess of caustic potassium solution and bromine were added to the resulting substance; As a result of the reaction, the color of bromine disappeared. Write the equations for the reactions described.
13. The insoluble substance formed when sodium hydroxide is added to a solution of ferric chloride was separated and dissolved in dilute sulfuric acid. Zinc dust was added to the resulting solution, the resulting precipitate was filtered and dissolved in concentrated hydrochloric acid. Write the equations for the reactions described.
14. Iron powder was dissolved in a large amount of dilute sulfuric acid and air was passed through the resulting solution, and then a gas with the smell of rotten eggs was passed through. The resulting insoluble salt was separated and dissolved in a hot solution of concentrated nitric acid. Write the equations for the reactions described.
15. Unknown substance A dissolves in concentrated hydrochloric acid, the dissolution process is accompanied by the release of gas with the smell of rotten eggs; after neutralizing the solution with alkali, a voluminous white (light green) precipitate is formed. When substance A is fired, two oxides are formed. One of them is a gas that has a characteristic pungent odor and discolors bromine water with the formation of two strong acids in solution. Write the equations for the reactions described.
16. A silver-gray metal that is attracted by a magnet was added to hot concentrated sulfuric acid and heated. The solution was cooled and caustic soda was added until the formation of an amorphous brown precipitate ceased. The precipitate was separated, calcined and dissolved in concentrated hydrochloric acid while heating. Write the equations for the reactions described.
17. The substance obtained by heating iron scale in a hydrogen atmosphere was added to hot concentrated acid and heated. The resulting solution was evaporated, the residue was dissolved in water and treated with a solution of barium chloride. The solution was filtered and a copper plate was added to the filtrate, which dissolved after some time. Write the equations for the reactions described.
18. A solution of iron (III) chloride was subjected to electrolysis. The brown precipitate formed during electrolysis was filtered and dissolved in a solution of sodium hydroxide, after which the amount of sulfuric acid necessary to form a clear solution was added. The product released at the anode was passed through a hot solution of potassium hydroxide. Write the equations for the reactions described.
19. Iron was burned in chlorine. The reaction product was dissolved in water and iron filings were added to the solution. After some time, the solution was filtered and sodium sulfide was added to the filtrate. The resulting precipitate was separated and treated with 20% sulfuric acid, obtaining an almost colorless solution. Write the equations for the reactions described.
20. A mixture of iron powder and a solid product obtained by the reaction of sulfur dioxide and hydrogen sulfide was heated without access to air. The resulting product was fired in air. The resulting solid reacts with the aluminum, releasing large amounts of heat. Write the equations for the reactions described.
21. Iron (III) oxide was fused with soda. The resulting product was added to water. The precipitate that formed was dissolved in hydroiodic acid. The released halogen was bound with sodium thiosulfate. Write the equations for the reactions described.
22. Chlorine reacted with a hot solution of potassium hydroxide. As the solution cooled, crystals of Berthollet salt precipitated. The resulting crystals were added to a solution of hydrochloric acid. the resulting simple substance reacted with metallic iron. The reaction product was heated with a new portion of iron. Write the equations for the reactions described.
23. Pyrite was fired, and the resulting gas with a pungent odor was passed through hydrogen sulfide acid. The resulting yellowish precipitate was filtered, dried, mixed with concentrated nitric acid and heated. The resulting solution gives a precipitate containing barium nitrate. Write the equations for the reactions described.
24. Iron filings were dissolved in dilute sulfuric acid, the resulting solution was treated with an excess of sodium hydroxide solution. The resulting precipitate was filtered and left in air until it acquired a brown color. The brown substance was calcined to constant mass. Write the equations for the reactions described.
25. Conducted electrolysis of a sodium chloride solution. Iron (III) chloride was added to the resulting solution. The precipitate that formed was filtered and calcined. The solid residue was dissolved in hydroiodic acid. Write the equations for the reactions described.
26. Potassium chlorate was heated in the presence of a catalyst, and a colorless gas was released. By burning iron in an atmosphere of this gas, iron oxide was obtained. It was dissolved in dilute hydrochloric acid. To the resulting solution was added a solution containing sodium dichromate and hydrochloric acid. Write the equations for the reactions described.
27. Iron was burned in chlorine. The resulting salt was added to the sodium carbonate solution, and a brown precipitate formed. This precipitate was filtered and calcined. The resulting substance was dissolved in hydroiodic acid. Write the equations for the reactions described.
28. Sulfur was fused with iron. The reaction product was treated with hydrochloric acid. The gas released was burned in excess oxygen. The combustion products were absorbed by an aqueous solution of iron (III) sulfate. Write the equations for the reactions described.
29. As a result of incomplete combustion of coal, a gas was obtained, in a current of which iron (III) oxide was heated. The resulting substance was dissolved in hot concentrated sulfuric acid. The resulting salt solution was treated with an excess of potassium sulfide solution. Write the equations for the reactions described.
30. Iron was burned in a chlorine atmosphere. The resulting substance was treated with an excess of sodium hydroxide solution. The resulting brown precipitate was filtered and calcined. The residue after calcination was dissolved in hydroiodic acid. Write the equations for the reactions described.
31. Iron was dissolved in dilute nitric acid. An excess of sodium carbonate solution was added to the resulting solution. The precipitate that separated was filtered and calcined. The resulting substance was ground into a fine powder along with aluminum and the mixture was set on fire. It burned, releasing a large amount of heat. Write the equations for the reactions described.
32. Iron powder was heated with sulfur powder. The reaction product was dissolved in hydrochloric acid, and excess alkali was added to the solution. The precipitate that formed was calcined in a nitrogen atmosphere. Write the equations for the reactions described.
33. Iron was burned in a chlorine atmosphere. The resulting salt was dissolved in water and a solution of potassium iodide was added to it. The precipitate of a simple substance was separated and divided into two parts. The first was treated with dilute nitric acid, and the second was heated in a hydrogen atmosphere. Write the equations for the reactions described.
34. Iron was dissolved in hydrochloric acid, and sodium hydroxide was added to the resulting solution until the precipitation stopped. Oxygen was first passed into the resulting reaction mass, and then hydroiodic acid was added until the precipitation stopped. Write the equations for the reactions described.
35. The precipitate obtained by reacting solutions of iron sulfate () and barium nitrate was filtered. The filtrate was treated with excess sodium hydroxide. The precipitate that formed was separated and calcined. The resulting substance was treated with an excess of hydrochloric acid solution. Write the equations for the reactions described.
Iron. Iron compounds.
1. 2Fe + 6H 2 SO 4 (conc.) Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Fe 2 (SO 4) 3 + 6NaOH = 2Fe(OH) 3 + 3Na 2 SO 4
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + Fe 3FeO
2. FeCl 3 + 3AgNO 3 = 3AgCl↓ + Fe(NO 3) 3
Fe(NO 3) 3 + 3KOH = Fe(OH) 3 ↓ + 3KNO 3
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 2Al 2Fe + Al 2 O 3
3. 2KMnO 4 + 16HCl = 2MnCl 2 + 2KCl + 5Cl 2 + 8H 2 O
2Fe + 3Cl 2 = 2FeCl 3
2FeCl 3 + 3Na 2 S = S↓ + 2FeS↓ + 6NaCl
S + 6HNO 3 (conc. hor.) = H 2 SO 4 + 6NO 2 + 2H 2 O
4. 2KMnO 4 + 16HBr = 2MnCl 2 + 2KCl + 5Br 2 + 8H 2 O
FeBr 3 + 3CsOH = Fe(OH) 3 ↓ + 3CsBr
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
5. 2FeCl 2 + 2H 2 O Fe + H 2 + Fe(OH) 2 + 2Cl 2
4FeS + 7O 2 2Fe 2 O 3 + 4SO 2
Ba(OH) 2 + SO 2 = BaSO 3 + H 2 O
6. 4FeCl 3 + 6H 2 O 2Fe + 3H 2 + 2Fe(OH) 3 + 6Cl 2
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe + 6HNO 3(conc.) Fe(NO 3) 3 + 3NO 2 + 3H 2 O
Cl 2 + 2KOH (cold) = KClO + KCl + H 2 O
7. FeCl 3 + 3KOH = Fe(OH) 3 ↓ + 3KCl
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + Na 2 CO 3 2NaFeO 2 + CO 2
2NaFeO 2 + 3NaNO 3 + 2NaOH 2Na 2 FeO 4 + 3NaNO 2 + H 2 O
8. 3FeO + 10HNO 3(dil.) 3Fe(NO 3) 3 + NO + 5H 2 O
9. FeCl 2 + 4HNO 3(conc.) = Fe(NO 3) 3 + NO 2 + 2HCl + H 2 O
2Fe(NO 3) 3 + 3K 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6KNO 3
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 3H 2 2Fe + 3H 2 O
10. 2FeCl 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6NaCl
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 3CO 2Fe + 3CO 2
11. Fe 3 O 4 + 10HNO 3 (conc.) = 3Fe (NO 3) 3 + NO 2 + 5H 2 O
2Fe(NO 3) 3 + Fe = 3Fe(NO 3) 2
Fe(NO 3) 2 + 2KOH = Fe(OH) 2 + 2KNO 3
4Fe(OH) 2 + O 2 + 2H 2 O = 4Fe(OH) 3
12. 2FeCl 3 + Fe = 3FeCl 2
2Fe(OH) 2 + H 2 O 2 = 2Fe(OH) 3 ↓
2Fe(OH) 3 + 3Br 2 + 10KOH = 2K 2 FeO 4 + 6KBr + 8H 2 O
13. FeCl 2 + 2NaOH = Fe(OH) 2 ↓ + 2NaCl
Fe(OH) 2 + H 2 SO 4 = FeSO 4 + 2H 2 O
FeSO 4 + Zn = ZnSO 4 + Fe
Fe + 2HCl = FeCl 2 + H 2
14. Fe + H 2 SO 4 (diluted) = FeSO 4 + H 2
4FeSO 4 + O 2 + 2H 2 SO 4 = 2Fe 2 (SO 4) 3 + 2H 2 O
Fe 2 (SO 4) 3 + 2H 2 S = FeSO 4 + 2S + FeS + 2H 2 SO 4
FeS + 12HNO3(conc.) = Fe(NO3)3 + 9NO2 + H2SO4 + 5H2O
15. FeS + 2HCl = FeCl 2 + H 2 S
FeCl 2 + 2NaOH = Fe(OH) 2 ↓ + 2NaCl
4FeS + 7O 2 = 2Fe 2 O 3 + 4SO 2
SO 2 + Br 2 + 2H 2 O = H 2 SO 4 + 2HBr
16. 2Fe + 6H 2 SO 4 (conc.) Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Fe 2 (SO 4) 3 + 6NaOH = 2Fe(OH) 3 ↓ + 3Na 2 SO 4
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
17. Fe 3 O 4 + 4H 2 3Fe + 4H 2 O
2Fe + 6H 2 SO 4 (conc.) Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Fe 2 (SO 4) 3 + BaCl 2 = FeCl 3 + BaSO 4 ↓
2FeCl 3 + Cu = 2FeCl 2 + CuCl 2
18. 4FeCl 3 + 6H 2 O 2Fe + 3H 2 + 2Fe(OH) 3 + 6Cl 2
Fe(OH) 3 + 3NaOH = Na 3
2Na 3 + 6H 2 SO 4 = Fe 2 (SO 4) 3 + 3Na 2 SO 4 + 12H 2 O
3Cl 2 + 6KOH (hor.) = KClO 3 + 5KCl + 3H 2 O
19. 2Fe + 3Cl 2 = 2FeCl 3
2FeCl 3 + Fe = 3FeCl 2
FeCl 2 + Na 2 S = FeS↓ + 2NaCl
FeS + H 2 SO 4 = FeSO 4 + H 2 S
20. SO 2 + 2H 2 S = 3S↓ + 2H 2 O
4FeS + 7O 2 2Fe 2 O 3 + 4SO 2
Fe 2 O 3 + 2Al 2Fe + Al 2 O 3
21. Fe 2 O 3 + Na 2 CO 3 2NaFeO 2 + CO 2
NaFeO 2 + 2H 2 O = Fe(OH) 3 + NaOH
2Fe(OH) 3 + 6HI = 2FeI 2 + I 2 + 6H 2 O
I 2 + 2Na 2 S 2 O 3 = 2NaI + Na 2 S 4 O 6
22. 3Cl 2 + 6KOH (hor.) = 5KCl + KClO 3 + 3H 2 O
KClO 3 + 6 HCl = KCl + 3Cl 2 + 3H 2 O
2Fe + 3Cl 2 = 2FeCl 3
2FeCl 3 + Fe = 3FeCl 2
23. 4FeS 2 + 11O 2 = 2Fe 2 O 3 + 8SO 2
SO 2 + 2H 2 S = 3S + 2H 2 O
H 2 SO 4 + Ba(NO 3) 2 = BaSO 4 ↓ + 2HNO 3
24. Fe + H 2 SO 4 = FeSO 4 + H 2
FeSO 4 + 2NaOH = Fe(OH) 2 ↓ + Na 2 SO 4
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
25. 2NaCl + 2H 2 O H 2 + 2NaOH + Cl 2
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 6HI (conc.) = 2FeI 2 + I 2 + 3H 2 O
26. 2KClO 3 2KCl + 3O 2
3Fe + 2O 2 = Fe 3 O 4
Fe 3 O 4 + 8HCl = FeCl 2 + 2FeCl 3 + 4H 2 O
6FeCl 2 + Na 2 Cr 2 O 7 + 14HCl = 6FeCl 3 + 2CrCl 3 + 2NaCl + 7H 2 O
27. 2Fe + 3Cl 2 = 2FeCl 3
2FeCl 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 3CO 2 + 6NaCl
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
FeS + 2HCl = FeCl 2 + H 2 S
2H 2 S + 3O 2 = 2SO 2 + 2H 2 O
Fe 2 (SO 4) 3 + SO 2 + 2H 2 O = 2FeSO 4 + 2H 2 SO 4
29. C + O 2 2CO
Fe 2 O 3 + 3CO 2Fe + 3CO 2
2Fe + 6H 2 SO 4 Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Fe 2 (SO 4) 3 + 3K 2 S = 2FeS + S + 3K 2 SO 4
30. 2Fe + 3Cl 2 = 2FeCl 3
FeCl 3 + 3NaOH = Fe(OH) 3 + 3NaCl
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 6HI = 2FeI 2 + I 2 + 3H 2 O
31. Fe + 4HNO 3 (diluted) = Fe(NO 3) 3 + NO + 2H 2 O
(N 2 O and N 2 are also accepted as a product of the reduction of HNO 3)
2Fe(NO 3) 3 + 3Na 2 CO 3 + 3H 2 O = 2Fe(OH) 3 ↓ + 6NaNO 3 + 3CO 2
2HNO 3 + Na 2 CO 3 = 2NaNO 3 + CO 2 + H 2 O
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 2Al 2Fe + Al 2 O 3
FeS + 2HCl = FeCl 2 + H 2 S
FeCl 2 + 2KOH = Fe(OH) 2 ↓ + 2KCl
Fe(OH) 2 FeO + H 2 O
33. 2Fe + 3Cl 2 = 2FeCl 3
2FeCl 3 + 2KI = 2FeCl 2 + I 2 + 2KCl
3I 2 + 10HNO 3 = 6HIO 3 + 10NO + 2H 2 O
34. Fe + 2HCl = FeCl 2 + H 2
FeCl 2 + 2NaOH = Fe(OH) 2 ↓ + 2NaCl
4Fe(OH) 2 + 2H 2 O + O 2 = 4Fe(OH) 3 ↓
Fe(OH) 3 + 6HI = 2FeI 2 + I 2 + 6H 2 O
35. Fe 2 (SO 4) 3 + 3Ba(NO 3) 2 = 3BaSO 4 ↓ + 2Fe(NO 3) 3
Fe(NO 3) 3 + 3NaOH = Fe(OH) 3 ↓ + 3NaNO 3
2Fe(OH) 3 Fe 2 O 3 + 3H 2 O
Fe 2 O 3 + 6HCl 2FeCl 3 + 3H 2 O
Zinc. Zinc compounds.
Zinc is a fairly active metal, but it is stable in air because it is covered with a thin layer of oxide, which protects it from further oxidation. When heated, zinc reacts with simple substances (the exception is nitrogen):
2Zn + О 2 2ZnО
Zn + Cl 2 ZnCl 2
3Zn + 2P Zn 3 P 2
as well as with non-metal oxides and ammonia:
3Zn + SO 2 2ZnO + ZnS
Zn + CO 2 ZnO + CO
3Zn + 2NH 3 Zn 3 N 2 + 3H 2
When heated, zinc oxidizes under the action of water vapor:
Zn + H 2 O (steam) ZnO + H 2
Zinc reacts with solutions of sulfuric and hydrochloric acids, displacing hydrogen from them:
Zn + 2HCl = ZnCl 2 + H 2
Zn + H 2 SO 4 = ZnSO 4 + H 2
How the active metal zinc reacts with oxidizing acids:
Zn + 2H 2 SO 4 (conc.) = ZnSO 4 + SO 2 + 2H 2 O
4Zn + 5H 2 SO 4 (conc.) = 4ZnSO 4 + H 2 S + 4H 2 O
Zn + 4HNO 3(conc.) → Zn(NO 4) 2 + 2NO 2 + 2H 2 O
4Zn + 10HNO 3(ultra dil.) = 4Zn(NO 3) 2 + NH 4 NO 3 + 3H 2 O
When zinc is fused with alkalis, zincate is formed:
Zn + 2NaOH (crystal) Na 2 ZnO 2 + H 2
Zinc dissolves well in alkali solutions:
Zn + 2KOH + 2H 2 O = K 2 + H 2
Unlike aluminum, zinc also dissolves in an aqueous solution of ammonia:
Zn + 4NH 3 + 2H 2 O = (OH) 2 + H 2
Zinc reduces many metals from solutions of their salts:
CuSO 4 + Zn = Zn SO 4 + Cu
Pb(NO 3) 2 + Zn = Zn(NO 3) 2 + Pb
4Zn + KNO 3 + 7KOH = NH 3 + 4K 2 ZnO 2 + 2H 2 O
4Zn + 7NaOH + 6H 2 O + NaNO 3 = 4Na 2 + NH 3
3Zn + Na 2 SO 3 + 8HCl = 3ZnCl 2 + H 2 S + 2NaCl + 3H 2 O
Zn + NaNO 3 + 2HCl = ZnCl 2 + NaNO 2 + H 2 O
II. Zinc compounds (zinc compounds are poisonous).
1) Zinc oxide.
Zinc oxide has amphoteric properties.
ZnO + 2HCl = ZnCl 2 + H 2 O
ZnO + 2NaOH Na 2 ZnO 2 + H 2 O
ZnO + Na 2 O Na 2 ZnO 2
ZnO + SiO 2 ZnSiO 3
ZnO + BaCO 3 BaZnO 2 + CO 2
Zinc is reduced from oxides by the action of strong reducing agents:
ZnO + C (coke) Zn + CO
ZnO + CO Zn + CO 2
2) Zinc hydroxide.
Zinc hydroxide has amphoteric properties.
Zn(OH) 2 + 2HCl = ZnCl 2 + 2H 2 O
Zn(OH) 2 + 2NaOH Na 2 ZnO 2 + 2H 2 O
Zn(OH) 2 + 2NaOH = Na 2
2Zn(OH) 2 + CO 2 = (ZnOH) 2 CO 3 + H 2 O
Zn(OH) 2 + 4(NH 3 H 2 O) = (OH) 2
Zinc hydroxide is thermally unstable:
Zn(OH) 2 ZnO + H 2 O
3) Salt.
CaZnO 2 + 4HCl (excess) = CaCl 2 + ZnCl 2 + 2H 2 O
Na 2 ZnO 2 + 2H 2 O = Zn(OH) 2 + 2NaHCO 3
Na 2 + 2CO 2 = Zn(OH) 2 + 2NaHCO 3
2ZnSO 4 2ZnO + 2SO 2 + O 2
ZnS + 4H 2 SO 4 (conc.) = ZnSO 4 + 4SO 2 + 4H 2 O
ZnS + 8HNO 3 (conc.) = ZnSO 4 + 8NO 2 + 4H 2 O
ZnS + 4NaOH + Br 2 = Na 2 + S + 2NaBr
Zinc. Zinc compounds.
1. Zinc oxide was dissolved in a solution of hydrochloric acid and the solution was neutralized by adding sodium hydroxide. The released white gelatinous substance was separated and treated with an excess of alkali solution, and the precipitate was completely dissolved. neutralization of the resulting solution with an acid, for example, nitric acid, leads to the re-formation of a gelatinous precipitate. Write the equations for the reactions described.
2. Zinc was dissolved in very dilute nitric acid and excess alkali was added to the resulting solution, obtaining a clear solution. Write the equations for the reactions described.
3. The salt obtained by reacting zinc oxide with sulfuric acid was calcined at a temperature of 800°C. The solid reaction product was treated with a concentrated alkali solution, and carbon dioxide was passed through the resulting solution. Write the equations for the reactions described.
4. Zinc nitrate was calcined, and the reaction product was treated with sodium hydroxide solution when heated. Carbon dioxide was passed through the resulting solution until the precipitation ceased, after which it was treated with an excess of concentrated ammonia, and the precipitate dissolved. Write the equations for the reactions described.
5. Zinc was dissolved in very dilute nitric acid, the resulting solution was carefully evaporated and the residue was calcined. The reaction products were mixed with coke and heated. Write the equations for the reactions described.
6. Several zinc granules were dissolved by heating in a solution of sodium hydroxide. Nitric acid was added to the resulting solution in small portions until a precipitate formed. The precipitate was separated, dissolved in dilute nitric acid, the solution was carefully evaporated and the residue was calcined. Write the equations for the reactions described.
7. Zinc metal was added to concentrated sulfuric acid. the resulting salt was isolated, dissolved in water, and barium nitrate was added to the solution. After separating the precipitate, magnesium shavings were added to the solution, the solution was filtered, the filtrate was evaporated and calcined. Write the equations for the reactions described.
8. Zinc sulfide was calcined. The resulting solid reacted completely with the potassium hydroxide solution. Carbon dioxide was passed through the resulting solution until a precipitate formed. The precipitate was dissolved in hydrochloric acid. Write the equations for the reactions described.
9. A certain amount of zinc sulfide was divided into two parts. One of them was treated with hydrochloric acid, and the other was fired in air. When the released gases interacted, a simple substance was formed. This substance was heated with concentrated nitric acid, and a brown gas was released. Write the equations for the reactions described.
10. Zinc was dissolved in a solution of potassium hydroxide. The released gas reacted with lithium, and hydrochloric acid was added dropwise to the resulting solution until precipitation stopped. It was filtered and calcined. Write the equations for the reactions described.
1) ZnO + 2HCl = ZnCl 2 + H 2 O
ZnCl 2 + 2NaOH = Zn(OH) 2 ↓ + 2NaCl
Zn(OH) 2 + 2NaOH = Na 2
Na 2 + 2HNO 3 (deficiency) = Zn(OH) 2 ↓ + 2NaNO 3 + 2H 2 O
2) 4Zn + 10HNO 3 = 4Zn(NO 3) 2 + NH 4 NO 3 + 3H 2 O
HNO 3 + NaOH = NaNO 3 + H 2 O
NH 4 NO 3 + NaOH = NaNO 3 + NH 3 + H 2 O
Zn(NO 3) 2 + 4NaOH = Na 2 + 2NaNO 3
3) ZnO + H 2 SO 4 = ZnSO 4 + H 2 O
2ZnSO 4 2ZnO + 2SO 2 + O 2
ZnO + 2NaOH + H 2 O = Na 2
4) 2Zn(NO 3) 2 2ZnO + 4NO 2 + O 2
ZnO + 2NaOH + H 2 O = Na 2
Na 2 + 2CO 2 = Zn(OH) 2 ↓ + 2NaHCO 3
Zn(OH) 2 + 4(NH 3 H 2 O) = (OH) 2 + 4H 2 O
5) 4Zn + 10HNO 3 = 4Zn(NO 3) 2 + NH 4 NO 3 + 3H 2 O
2Zn(NO 3) 2 2ZnO + 4NO 2 + O 2
NH 4 NO 3 N 2 O + 2H 2 O
ZnO + C Zn + CO
6) Zn + 2NaOH + 2H 2 O = Na 2 + H 2
Na 2 + 2HNO 3 = Zn(OH) 2 ↓ + 2NaNO 3 + 2H 2 O
Zn(OH) 2 + 2HNO 3 = Zn(NO 3) 2 + 2H 2 O
2Zn(NO 3) 2 2ZnO + 4NO 2 + O 2
7) 4Zn + 5H 2 SO 4 = 4ZnSO 4 + H 2 S + 4H 2 O
ZnSO 4 + Ba(NO 3) 2 = Zn(NO 3) 2 + BaSO 4
Zn(NO 3) 2 + Mg = Zn + Mg(NO 3) 2
2Mg(NO 3) 2 2Mg(NO 2) 2 + O 2
8) 2ZnS + 3O 2 = 2ZnO + 2SO 2
ZnO + 2NaOH + H 2 O = Na 2
Na 2 + CO 2 = Zn(OH) 2 + Na 2 CO 3 + H 2 O
Zn(OH) 2 + 2HCl = ZnCl 2 + 2H 2 O
9) ZnS + 2HCl = ZnCl 2 + H 2 S
2ZnS + 3O 2 = 2ZnO + 2SO 2
2H 2 S + SO 2 = 3S + 2H 2 O
S + 6HNO 3 = H 2 SO 4 + 6NO 2 + 2H 2 O
10) Zn + 2KOH + 2H 2 O = K 2 + H 2
H2 + 2Li = 2LiH
K 2 + 2HCl = 2KCl + Zn(OH) 2 ↓
Zn(OH) 2 ZnO + H 2 O
Copper and copper compounds.
Copper is a chemically low-active metal; it does not oxidize in dry air and at room temperature, but in humid air, in the presence of carbon monoxide (IV), it becomes covered with a green coating of hydroxycopper (II) carbonate.
2Cu + H2O + CO2 = (CuOH)2CO3
When heated, copper reacts with fairly strong oxidizing agents,
with oxygen, forming CuO, Cu 2 O depending on the conditions:
4Cu + O 2 2Cu 2 O 2Cu + O 2 2CuO
With halogens, sulfur:
Cu + Cl 2 = CuCl 2
Сu + Br 2 = CuBr 2
Copper dissolves in oxidizing acids:
when heated in concentrated sulfuric acid:
Cu + 2H 2 SO 4 (conc.) CuSO 4 + SO 2 + 2H 2 O
without heating in nitric acid:
Cu + 4HNO 3(conc.) = Cu(NO 3) 2 + 2NO 2 + 2H 2 O
3Cu + 8HNO 3(dissolved..) = 3Cu(NO 3) 2 + 2NO 2 + 4H 2 O
3Cu + 2HNO3 + 6HCl = 3CuCl2 + 2NO + 4H2O
Copper is oxidized by nitrogen oxide (IV) and iron salts (III)
2Cu + NO 2 = Cu 2 O + NO
2FeCl 3 + Cu = 2FeCl 2 + CuCl 2
Copper displaces metals to the right in the voltage series from solutions of their salts:
Hg(NO 3) 2 + Cu = Cu(NO 3) 2 + Hg
II. Copper compounds.
1) Oxides.
Copper(II) oxide
In the laboratory, copper (II) oxide is obtained by oxidation of copper when heated, or by calcination (CuOH) 2 CO 3, Cu(NO 3) 2:
Copper oxide exhibits weakly expressed amphoteric properties ( with predominance main). CuO interacts with acids:
СuO + 2HBr = CuBr 2 + H 2 O
CuO + 2HCl = CuCl 2 + H 2 O
CuO + 2H + = Cu 2+ + H 2 O
3CuO + 2NH 3 3Cu + N 2 + 3H 2 O
СuO + C = Cu + CO
3CuO + 2Al = 3Cu + Al 2 O 3
Copper(I) oxide
In the laboratory it is obtained by reducing freshly precipitated copper (II) hydroxide, for example, with aldehydes or glucose:
CH 3 CHO + 2Cu(OH) 2 CH 3 COOH + Cu 2 O↓ + 2H 2 O
CH 2 OH (CHOH) 4 CHO + 2Cu(OH) 2 CH 2 OH (CHOH) 4 COOH + Cu 2 O↓ + 2H 2 O
Copper(I) oxide has main properties. When copper (I) oxide is treated with hydrohalic acid, copper (I) halides and water are obtained:
Cu 2 O + 2HCl = 2CuCl↓ + H 2 O
When Cu 2 O is dissolved in oxygen-containing acids, for example, in sulfuric solution, copper (II) salts and copper are formed:
Cu 2 O + H 2 SO 4 (diluted) = CuSO 4 + Cu + H 2 O
In concentrated sulfuric and nitric acids, only salts (II) are formed.
Cu 2 O + 3H 2 SO 4 (conc.) = 2CuSO 4 + SO 2 + 3H 2 O
Cu 2 O + 6HNO 3 (conc.) = 2Cu(NO 3) 2 + 2NO 2 + 3H 2 O
5Cu2O + 13H2SO4 + 2KMnO4 = 10CuSO4 + 2MnSO4 + K2SO4 + 13H2O
Stable copper (I) compounds are insoluble compounds (CuCl, Cu 2 S) or complex compounds +. The latter are obtained by dissolving copper (I) oxide and copper (I) chloride in a concentrated solution of ammonia:
Cu 2 O + 4NH 3 + H 2 O = 2OH
CuCl + 2NH 3 = Cl
Ammonia solutions of copper (I) salts react with acetylene:
СH ≡ CH + 2Cl → Сu–C ≡ C–Cu + 2NH 4 Cl
In redox reactions, copper (I) compounds exhibit redox duality
Cu 2 O + CO = 2Cu + CO 2
Cu 2 O + H 2 = 2Cu + H 2 O
3Cu 2 O + 2Al = 6Cu + Al 2 O 3
2Cu2O + O2 = 4CuO
2) Hydroxides.
Copper(II) hydroxide.
Copper(II) hydroxide exhibits weakly expressed amphoteric properties (with a predominance of main). Cu(OH) 2 interacts with acids:
Cu(OH) 2 + 2HBr = CuBr 2 + 2H 2 O
Cu(OH) 2 + 2HCl = CuCl 2 + 2H 2 O
Cu(OH) 2 + 2H + = Cu 2+ + 2H 2 O
Copper(II) hydroxide easily reacts with ammonia solution, forming a blue-violet complex compound:
Сu(OH) 2 + 4(NH 3 H 2 O) = (OH) 2 + 4H 2 O
Cu(OH) 2 + 4NH 3 = (OH) 2
When copper (II) hydroxide reacts with concentrated (more than 40%) alkali solutions, a complex compound is formed:
Cu(OH) 2 + 2NaOH (conc.) = Na 2
When heated, copper(II) hydroxide decomposes:
Сu(OH) 2 CuO + H 2 O
3) Salt.
Copper (I) salts.
In redox reactions, copper(I) compounds exhibit redox duality. As reducing agents they react with oxidizing agents:
CuCl + 3HNO 3(conc.) = Cu(NO 3) 2 + HCl + NO 2 + H 2 O
2CuCl + Cl 2 = 2CuCl 2
4CuCl + O 2 + 4HCl = 4CuCl 2 + 2H 2 O
2CuI + 4H 2 SO 4 + 2MnO 2 = 2CuSO 4 + 2MnSO 4 + I 2 + 4H 2 O
4CuI + 5H 2 SO 4 (conc. hor.) = 4CuSO 4 + I 2 + H 2 S + 4H 2 O
Cu 2 S + 8HNO 3 (conc. cold) = 2Cu(NO 3) 2 + S + 4NO 2 + 4H 2 O
Cu 2 S + 12HNO 3 (conc. cold) = Cu (NO 3) 2 + CuSO 4 + 10NO 2 + 6H 2 O
For copper (I) compounds, a disproportionation reaction is possible:
2CuCl = Cu + CuCl 2
Complex compounds of type + are obtained by dissolving in a concentrated ammonia solution:
CuCl + 3NH 3 + H 2 O → OH + NH 4 Cl
Copper(II) salts
In redox reactions, copper (II) compounds exhibit oxidizing properties:
2CuCl 2 + 4KI = 2CuI + I 2 + 4HCl
2CuCl 2 + Na 2 SO 3 + 2NaOH = 2CuCl + Na 2 SO 4 + 2NaCl + H 2 O
5CuBr 2 + 2KMnO 4 + 8H 2 SO 4 = 5CuSO 4 + K 2 SO 4 + 2MnSO 4 + 5Br 2 + 8H 2 O
CuSO 4 + Fe = FeSO 4 + Cu
CuS + 8HNO 3 (conc. hor..) = CuSO 4 + 8NO 2 + 4H 2 O
CuS + 2FeCl 3 = CuCl 2 + 2FeCl 2 + S
2CuS + 3O 2 2CuO + 2SO 2
CuS + 10HNO 3(conc.) = Cu(NO 3) 2 + H 2 SO 4 + 8NO 2 + 4H 2 O
2CuCl 2 + 4KI = 2CuI + I 2 ↓ + 4KCl
CuBr 2 + Na 2 S = CuS↓ + 2NaBr
Cu(NO 3) 2 + Fe = Fe(NO 3) 2 + Cu
CuSO 4 + Cu + 2NaCl = 2CuCl↓ + Na 2 SO 4
2Cu(NO 3) 2 + 2H 2 O 2Cu + O 2 + 4HNO 3
CuSO 4 + 2NaOH = Cu(OH) 2 ↓ + Na 2 SO 4
2Cu(NO 3) 2 2CuO + 4NO 2 + O 2
CuCl 2 + 4NH 3 = Cl 2
(CuOH) 2 CO 3 2CuO + CO 2 + H 2 O
Na 2 + 4HCl = 2NaCl + CuCl 2 + 4H 2 O
2Cl + K 2 S = Cu 2 S + 2KCl + 4NH 3
When mixing solutions, hydrolysis occurs at both the weak base cation and the weak acid anion:
2CuSO 4 + Na 2 SO 3 + 2H 2 O = Cu 2 O + Na 2 SO 4 + 2H 2 SO 4
2CuSO 4 + 2Na 2 CO 3 + H 2 O = (CuOH) 2 CO 3 ↓ + 2Na 2 SO 4 + CO 2
Copper and copper compounds.
1) A direct electric current was passed through a solution of copper (II) chloride using graphite electrodes. The electrolysis product released at the cathode was dissolved in concentrated nitric acid. The resulting gas was collected and passed through a sodium hydroxide solution. The gaseous electrolysis product released at the anode was passed through a hot sodium hydroxide solution. Write the equations for the reactions described.
2) The substance obtained at the cathode during the electrolysis of molten copper (II) chloride reacts with sulfur. The resulting product was treated with concentrated nitric acid, and the liberated gas was passed through a solution of barium hydroxide. Write the equations for the reactions described.
3) The unknown salt is colorless and turns the flame yellow. When this salt is slightly heated with concentrated sulfuric acid, the liquid in which the copper dissolves is distilled off; the latter transformation is accompanied by the release of brown gas and the formation of a copper salt. During the thermal decomposition of both salts, one of the decomposition products is oxygen. Write the equations for the reactions described.
4) When a solution of salt A interacted with an alkali, a gelatinous, water-insoluble substance of blue color was obtained, which was dissolved in colorless liquid B to form a blue solution. The solid product remaining after careful evaporation of the solution was calcined; in this case, two gases were released, one of which is brown in color, and the second is part of the atmospheric air, and a black solid substance remains, which dissolves in liquid B to form substance A. Write the equations for the described reactions.
5) Copper turnings were dissolved in dilute nitric acid, and the solution was neutralized with caustic potash. The released blue substance was separated, calcined (the color of the substance changed to black), mixed with coke and calcined again. Write the equations for the reactions described.
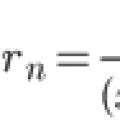 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A