The influence of motor transport on flora and fauna. Man and natural resources
Soil is a necessary and irreplaceable substrate in which plants strengthen their roots and from which they draw moisture and mineral nutrients. The abundance of soil conditions at all levels of soil cover organization determines the formation in the soil of a huge amount of different types habitats, which determines the diversity of organisms living in the soil. That is why the role of soil in the formation and preservation of biological diversity is great. On the other hand, the flows of all elements in the biosphere pass through the soil, which, through specific mechanisms, regulates their direction and intensity.
Microorganisms carry out oxidation and reduction inorganic compounds, transforming them into a more or, conversely, less digestible form. The assimilation of atmospheric nitrogen by microorganisms, which is almost the only source of nitrogen compounds in the soil, is extremely important. One of the characteristics of microorganisms is their production of biotic substances, such as vitamins and hormones, which promote plant growth. Many higher plants live in symbiosis with fungi, forming mycorrhizae or fungal roots. Epiphytic microflora develops on the aboveground organs of plants, which contribute to plant growth by supplying them with hormonal substances. In general, the growth of higher plants is possible even on a sterile mineral nutrient medium, but in the presence of microorganisms it occurs more intensively.
The normal microflora of plants is represented by rhizosphere and epiphytic microbes. The zone of soil in contact with the root system of a plant is called the rhizosphere, and the microorganisms that develop in this zone are called rhizosphere. Conventionally, a distinction is made between the near and distant rhizosphere. The near one is located directly on the surface of the roots and is removed along with them, the distant one begins at a distance of several millimeters from the roots and spreads within a radius of 50 cm from them. The number of microorganisms in the near and distant rhizosphere is different: on the surface of the roots there are from 50 million to 10 billion, at a distance of 15 cm from the roots - up to 5 million in 1 g of soil. The number of microorganisms in the rhizosphere is 100 times greater than in the soil where plants do not grow, which is associated with the release of various nutrients by plant roots. In turn, soil microbes have a beneficial effect on plant life. This is due to their mineralization organic matter and plant residues, the formation of vitamins, amino acids, enzymes and other growth factors that enhance enzymatic processes in plants. These processes enhance root nutrition and intensify plant metabolism, and also play an antagonistic role against phytopathogenic microorganisms.
The qualitative and quantitative composition of the rhizosphere microflora is specific for each plant species. The bulk of the root microflora is represented by non-spore-bearing bacteria of the genus Pseudomonas, mycobacteria and fungi, mainly basidiomycetes, less often phycomycetes, ascomycetes. These fungi form a symbiosis with plant roots called mycorrhiza. Depending on the morphological characteristics of the cohabitation of fungi with plants, ectotrophic and endotrophic mycorrhizae are distinguished. Ectotrophic - associations in which the fungus does not penetrate into the roots, but settles on their surface, forming a kind of cover of mycelium. In endotrophic mycorrhizae, the fungal mycelium is located in the cells of the plant root cortex, where it forms clusters in the form of balls. Higher plants, being the main source of nutrients for the predominant microbial population of heterotrophic soils, have a significant impact on microbial cenoses. The zones immediately adjacent to the roots of living plants are areas of active development of microorganisms. This is due, first of all, to the secretions of organic substances synthesized by plants from the roots.
ECOLOGICAL PROBLEMS OF MOTOR TRANSPORT
There are two main directions for improving modern transport power plants:
Rational use of fuel;
Reducing the harmful impact of vehicles on the environment.
Balance of relative reduced emissions of internal combustion engines for individual components: CO - 5%; soot - 2%; СНх - 1%; SO2- 8%; NOx - 70%; lead - 14%.
Negative environmental consequences of motorization:
Pollution environment: ingredients®air, water, soil.
Environmental pollution: parametric®noise, heat, electromagnetic radiation; vibration.
Environmental pollution associated with the consumption of resources, labor costs, reduction of habitats, and death of living organisms.
The harmlessness of a car can be ensured by traffic safety, reduction of noise from vehicles, and reduction of environmentally polluting ingredients.
Traffic safety is the brake mechanisms, the parameters of which characterize the stability and controllability of the car. These are visibility, alarm efficiency, head restraints, seat belts, energy-absorbing steering column, and safety body parts.
Noise reduction is the quietness of the engine, gearbox, final drive, tire brakes, tightness of body joints, stability and quietness during vehicle operation.
Reduction of polluting ingredients. Complete combustion of fuel in the internal combustion engine, in all operating modes, absence of toxic components in exhaust gases (EG), presence and operation of exhaust gas neutralizers, prevention of crankcase gases from entering the atmosphere. Ultimately, the measures presented are the performance of the vehicle and its safety.
CAR IS A SOURCE OF ENVIRONMENTAL POLLUTION
Harmful and toxic substances contained in the exhaust gases of automobile engines may not undergo any changes in the atmosphere for a long time and can be transported over significant distances. In addition, primary pollutants in the atmosphere, under appropriate conditions, can interact with each other, forming new toxic or harmful substances, for example, sulfates, nitrates, ozone, acids, photooxidants, etc.
Sulfur and nitrogen oxides, being in the atmospheric air for up to 2-5 days and moving with the air flow over a distance of 1000 km, can turn into acids:
SO2+NO®SO3(H2O)®H2SO3.
H2SO3+ O2® SO3+HO2·.
H2SO3+OH·® H2SO4.
NO+ O3® NO2+O2.
The main atmospheric pollutants include sulfur dioxide, suspended particles, CO, CO2, NOx, photo-oxidants and reactive hydrocarbons, lead, mercury, cadmium, chlorinated organic compounds, petroleum products, microtoxins, ammonia, freons, metals, radioactive substances, etc.
The most toxic of chemical substances: mercury, arsenic, lead, zinc, cadmium, sulfur compounds, hydrocarbons (polycyclic aromatic hydrocarbons-PAHs). By contaminating the air and water, they cause poisoning, disorder nervous system, metabolic disorders, cancer. Human diseases are also caused by increased noise levels, vibration, and electromagnetic radiation.
Harmful effects for plants and animals are also associated with pollution of the natural environment with toxic substances: gases (H2S, HF, O3, NO2, Cl2), aerosols (HCl, H2SO4), heavy metals, inorganic salts, and petroleum products.
Petroleum products cause the death of microorganisms and phytoplankton in water bodies, negatively affect the morphological and physiological functions of plants, causing their diseases (chlorosis, necrosis), lack or excess of certain chemical elements in soil and water.
Living organisms are sensitive to any changes in the environment. The CO2 contained in exhaust gas, as well as the heat from power plants, contributes to the formation of the greenhouse effect - climate warming on a global scale.
SOURCES OF HARMFUL SUBSTANCES AND THEIR INFLUENCE ON THE HUMAN BODY
The main source of air pollution today is spark ignition engines. However, reducing the toxicity of automobile diesel engines also deserves serious attention.
SOURCES OF HARMFUL AND TOXIC EMISSIONS
In any power plant (engine), polluting emissions are generated during fuel combustion. Liquid fuel for internal combustion engines contains a sufficient amount of elements C, H and a large number of O, N, S. Air contains N2 - 78.03%; O2 - 20.99%; CO2- 0.03%; inert gases - 0.04%.
To ensure the combustion process, a working mixture consisting of one part fuel and 15 parts air is supplied to the internal combustion engine. Therefore, harmful and toxic components are formed in the exhaust gas as a result of combustion of the working mixture.
In total, the exhaust gas of automobile internal combustion engines contains about 280 components, which, according to their chemical properties and the nature of their impact on the biosphere, are divided into non-toxic (H2O, H2, O2, N2), harmful - CO2 and toxic (CO, NOx, CHx, SO2, H2S, aldehydes , soot, etc.).
The main sources of harmful and toxic emissions include crankcase gases, fuel vapors, and the fuel tank.
Fuel vapor (CxHy) is fuel vapor that enters the atmosphere from fuel tanks, elements of the engine power system: joints, hoses, etc. They consist of hydrocarbons. Due to the high viscosity of diesel fuel, diesel engines emit less hydrocarbon vapors. There are also vapors from fuels and lubricants and special liquids - oil leakage, evaporation of antifreeze.
Crankcase gases are a mixture of gases penetrating through the leaks of the piston rings from the combustion chamber into the crankcase, and oil vapors located in the crankcase, and then entering the environment. The mixture of these gases greatly irritates the mucous membrane of the respiratory system.
Exhaust gases (CO, CHx, NOx, soot, etc.) are a mixture of gaseous products of complete (incomplete) combustion of fuel, excess air and various microimpurities (gaseous, liquid and solid particles coming from the engine cylinders into its exhaust system) .
Summarizing the data from the tables, we can conclude that a gasoline engine emits approximately 7 times more CO than a diesel engine, and approximately 3 times more aldehydes than a diesel engine. The emissions of the remaining components of these engines are almost identical. However, diesel emits more (about 10-15 times) SO2.
CONTENT OF HARMFUL AND TOXIC EMISSIONS, THEIR EFFECT ON THE HUMAN BODY
Harmful and toxic emissions are conventionally divided into regulated and unregulated. They act on the human body in different ways. Toxic emissions: CO, NOx, CHx, RxCHO, SO3, soot, smoke.
CO-carbon monoxide is a colorless and odorless gas, lighter than air. It forms on the surface of the piston and on the wall of the cylinder, in which activation does not occur due to intense heat removal in the wall, poor fuel atomization and dissociation of CO2 into CO and O2 at high temperatures.
C+1/2CO2=CO.
During diesel operation, the CO concentration is insignificant (0.1-0.2%). In a gasoline engine, when idling and at low loads, the CO content reaches 5-8% (due to operation on enriched mixtures?).
CO causes nervous system disorders, headaches, weight loss, and vomiting. This happens because CO changes the composition of the blood and reduces the formation of hemoglobin, interfering with the process of oxygen saturation in the body. Hemoglobin combined with carbon monoxide is called carboxyhemoglobin. Hemoglobin bound to oxygen is called oxyhemoglobin.
People with elevated carboxyhemoglobin levels experience two important symptoms. This is a decrease in the ability to perceive signals coming from the external environment and disruption of thinking processes.
NOx (nitrogen oxides) - all nitrogen oxides are physiologically active and belong to the third hazard class. MPC (in terms of NO2) - 5 mg/m3.
N2 is an inert gas that reacts actively with oxygen at high temperatures. NOx emissions from exhaust gas depend on the ambient temperature. The greater the engine load, the higher the temperature in the combustion chamber, and accordingly the emission of nitrogen oxides increases. The temperature in the combustion zone (combustion chamber) largely depends on the composition of the mixture. A mixture that is too lean or rich produces less heat during combustion. The combustion process slows down and is accompanied by large heat losses in the wall, i.e., under such conditions, less NOx is released, and emissions increase when the mixture composition is close to stoichiometric. For diesel engines, the NOx composition depends on the fuel injection angle and the auto-ignition delay time.
Nitrogen oxides irritate the mucous membrane of the eyes and nose and destroy the lungs. In the respiratory tract, nitrogen oxides react with moisture. Nitrogen oxides destroy the ozone layer.
It is believed that the toxicity of NOx is 10 times greater than the toxicity of CO.
Hydrocarbons (CxHy) are conventionally ethane, methane, etc. Exhaust gas contains 200 different hydrocarbons.
In diesel engines, CxHy are formed in the combustion chamber due to low mixture homogeneity, i.e. in cases of a rich mixture, where the flame goes out, where there is weak air turbulence, low temperature, poor atomization.
CxHy have an unpleasant odor, CxHy in the form of vapor (gasoline) is also toxic.
ICEs emit large amounts of CxHy when idling due to poor turbulence and reduced combustion rate.
CxNy irritate the eyes and nose and are very harmful to flora and fauna. Saturated hydrocarbons have a narcotic effect on the human body.
Unsaturated hydrocarbons. Olefins cause lacrimation, coughing, and disturbances in the functioning of the nervous system. Reacting with nitrogen oxides under the influence of sunlight, they form biologically active substances that cause irritation to the respiratory system and also cause damage to flora and fauna.
Polycyclic aromatic hydrocarbons. PAHs are divided into 4 groups based on their degree of carcinogenicity:
Strong carcinogens are benzo-a-pyrene, dibenz-a-pyrene;
Moderate carcinogens - benz-a-fluoratene;
Non-carcinogens - coronene, pyrene.
PAHs gradually accumulate in the human body to critical concentrations and stimulate the formation of malignant tumors.
Aldehydes. Organic compounds with the general chemical formula RxCHO, containing in the molecule a carbonyl group bonded to a carbon atom and a hydrocarbon radical (R=CH3·, C2H5·, etc.). Of the aldehydes, EG contains mainly formaldehydes and acrolein. Aldehydes are formed when fuel is burned at low temperatures or the mixture is very lean, and also as a result of oxidation of the thin layer of oil on the cylinder wall.
Formaldehyde is a colorless gas with a pungent and unpleasant odor, irritates the eyes and upper respiratory tract, and affects the central nervous system.
Acrolein. A colorless, highly volatile liquid that also has a strong irritant effect.
Smoke. Opaque gas, smoke can be white, blue, black. White and blue smoke is a mixture of fuel in the form of droplets with a microscopic amount of vapor; is formed due to incomplete combustion and subsequent condensation of the fuel.
White smoke is produced when the engine is idling, after the engine has warmed up. White color disappears. The difference between white smoke and blue smoke is determined by the size of the fuel potassium. The particle size of blue smoke is 0.001-0.1 microns, white smoke is more than 0.1 microns up to 100 microns. In this case, white smoke is formed in the engine temperature range of 100-3000C, and blue smoke in the range of 300-7000C. Blue color smoke is also typical for smoke from oil.
Soot (black smoke). It is a shapeless body without a crystal lattice. In the exhaust gas of a diesel engine, soot is particles (dispersed particles) with a size of 0.3-100 microns. Soot formation depends on temperature, combustion chamber pressure, fuel type, and fuel-air ratio.
When soot gets into the respiratory tract, it causes chronic diseases, pollutes the air, impairs visibility and adsorbs strong carcinogenic substances on its surface, for example, benzo-a-pyrene.
PbхOy (lead oxides). Currently, leaded gasoline, which is a major source of lead oxide pollutants, is not used as a fuel. However, the lead content in gasoline according to GOST 2002 is 0.005 g/dm3. Therefore, during long-term use of such fuel, lead-containing compounds are formed. Lead oxides accumulate in the human body, entering it through animal and plant foods and drinking water. Aerosol compounds containing lead, like lead oxides, cause organ poisoning and disrupt the functions of the neuromuscular systems and brain. Lead is poorly excreted from the body, accumulating in it to concentrations dangerous to human health and life. Lead compounds accumulate in plants.
Photochemical air pollution. Photochemical reactions require light energy. Some pollutants, nitrogen oxides and hydrocarbons, undergo photochemical reactions. As a result of such reactions, new air pollutants are formed - ozone, aldehydes, as well as very specific organic compounds. Levels of photochemical air pollution are closely related to traffic patterns in the morning and evening. There are peak emissions of nitrogen oxides and hydrocarbons at these times of day. It is these compounds that react with each other that cause photochemical air pollution. Peroxyacyl nitrates (PAN) are formed. Ozone reacts with hydrocarbons to form an aldehyde.
SO2- (sulfur oxide). Formed during engine operation from fuel obtained from sulfur oil (especially in diesel engines). These emissions irritate the eyes and respiratory organs. High concentrations of SO2 and its derivatives cause serious damage to vegetation; many materials are destroyed by droplets of sulfuric acid. The benchmark for the effect of sulfuric acid on vegetation is the reddish-brown color of leaves and needles and the falling of leaves and needles.
INFLUENCE OF MOTOR TRANSPORT ON FLORA AND FAUNA
Pollution of the environment with toxic exhaust gas components leads to large economic losses on the farm, since toxic substances cause disturbances in plant growth, which leads to decreased yields and losses in livestock production.
There are also problems of soil pollution from vehicle waste.
EMISSION OF TOXIC COMPONENTS OF VEHICLES IN TRAFFIC FLOW
Fuel consumption and emission of toxic components. The perfection of car design is assessed by a set of operational properties, among which one of the most important is fuel efficiency. Fuel efficiency of a vehicle refers to its ability to use the minimum possible amount of fuel when performing transport work.
Fuel efficiency indicators are regulated by GOSTs. Their list includes control fuel consumption, fuel characteristics of the vehicle in steady state, fuel consumption in the highway and urban cycles on the roads, in the urban cycle on stands with running drums, as well as fuel-speed characteristics on the highway and hilly roads.
The total fuel consumption Q is determined by the energy losses in the engine (Qmotor) and transmission (Qtr), as well as the total resistance to movement, which consists of rolling resistance (Qf), aerodynamic resistance (Qw), resistance to energy forces (Qa) and lifting resistance (Qi ).
Fuel balance:
Q= Qmot+ Qtr+ Qf+ Qw+ Qi+ Qa.
When a small class car moves along a horizontal section of the road at a speed of 60 km/h, the specific weight of the components is distributed as follows: Qmotor = 65%; Qtr=9%; Qf=16%; Qw=10%.
As a meter for operational fuel consumption q, the ratio of total fuel consumption Q to the distance traveled S is used:
The figure shows the dependence of the operational fuel consumption of a middle-class passenger car on a city highway and a section of a country road on the speed of communication v=S/t (t is the time of movement of the vehicle).
Curve 1 represents fuel consumption depending on the steady speed, and the shaded area corresponds to fuel consumption when driving at an economical speed.
In city conditions, a car moves mainly in acceleration and deceleration modes, and the combination of these phases can be very diverse. All this makes it impossible to drive in urban conditions at economical speeds and leads to additional fuel consumption (the zone between curves 1 and 2).
Let us consider the movement of two cars along a section of a city highway in free conditions. Let the first car cover the section at a speed of 60 km/h almost unhindered. The fuel consumption of the first car is:
where qL is specific fuel consumption, l/km; l is the length of the section, km.
The fuel consumption of the second car Q2 will be the sum of the fuel consumption for acceleration Qр, for braking Qт, for idling Qхх and for movement at a relatively constant speed Qv:
Q2= Qр+ Qт+ Qхх+ Qv.
Fuel consumption Q2* is equal to:
where D0 is the additional fuel consumption associated with vehicle stops, l.
Consumption D0 will be determined by the number of stops O and idling time tхх:
D0= q0∙O+ qхх∙tхх,
where q0 is additional fuel consumption per stop, l/h; qхх - fuel consumption at idle, l/h.
Fuel consumption q0 depends on the intensity and final acceleration speed Vр. In addition to the acceleration speed, additional fuel consumption on stopping is affected by the number of the car in the queue and its composition.
The increase in fuel consumption of the i-th car is taken into account by the Koch priority coefficient.
The unevenness of the speed regime for a continuous highway is estimated with sufficient accuracy by the parameters of the traffic flow, i.e., the parameters of the final acceleration speed, the queuing coefficient, and the delay coefficient.
However, this parameter is inaccurate when applied to a city highway. Therefore, the velocity gradient parameter has also been introduced. The velocity gradient Iv reflects the relative proportion of unsteady motion modes per unit time. In addition, Iv characterizes the level of transport load, atmospheric pollution with toxic exhaust gas components, and fuel consumption.
In free motion conditions, the speed gradient values are small and the speed is highest. As the load level increases, the mutual influence of cars increases, the drivers of which are forced to constantly react to changes in the road situation, the unevenness of traffic increases and the speed decreases. This leads to an increase in specific consumption figures. Calculation of the speed gradient is possible based on the spatio-temporal characteristics of the driving mode using the “floating” car method.
The figure shows the dependence of changes in fuel consumption during continuous movement on the speed gradient.
For all types of vehicles, an increase in traffic density leads to an increase in the speed gradient.
The values of specific fuel consumption depending on the state of traffic flow are given in the table.
Additional fuel consumption in the intersection area by the flow of cars is largely determined by the duration of the traffic light control cycle.
A fairly wide range of changes in fuel consumption is explained by the variety of traffic conditions in cities, so in each specific case the effectiveness of a certain measure can be assessed if all parameters of traffic flow are taken into account.
Reducing fuel consumption and, consequently, harmful emissions from vehicles is achieved:
Reducing the number of intersections of transport and pedestrian flows.
Reducing the level of highway congestion.
Optimization of the composition of the traffic flow.
Speed optimization.
Optimization of the control cycle.
Implementation of ASUD.
Field surveys of air pollution on highways. These surveys are necessary to assess the existing state of the traffic management system, the state of the environment, justify measures to improve them, adjust parameters for managing traffic flows and organizing their movement, developing the volume and priority of reconstruction of highways.
Preliminary observations are carried out using mobile laboratories, which per hour of work carry out 2-3 measurements at different (but nearby) points along the route. Combined methods are used, i.e., the characteristics of traffic flow are determined in all areas of interest. Air pollution is measured at specific locations only at a subset of them. At other points, the concentrations of harmful impurities are determined by calculation. The location for air sampling during the stretch is chosen at the edge of the roadway (at curb level). When taking air samples in a container, the meter is located on the lawn or sidewalk. When using a mobile laboratory, the car is parked on the lawn. The observation point (air sampling) should be located no closer than 30 m from the pedestrian crossing, car parks, and public transport stops. It is impossible to determine atmospheric air pollution by harmful components of vehicle exhaust gases during precipitation - rain or snow, as well as during fog or snowstorms.
To determine the concentrations of pollutants, laboratory and express methods are used. Express methods are based on pumping air through indicator tubes using a hand aspirator. Laboratory methods are divided into two types: determination of impurity concentrations directly at the observation site and sampling of air in containers with subsequent analysis of samples in the laboratory.
Outlier Definition harmful substances transport flows. A car pollutes the air with substances that are emitted with exhaust and crankcase gases and enter the atmosphere as a result of combustion and evaporation of fuel. At the same time, the bulk of harmful emissions comes from exhaust gases. Biologically active are CO, NOx, CxHy, aldehydes, smoke, soot, hydrocarbon compounds of the carcinogenic group.
The table shows the performance characteristics of the car engine and toxicity indicators in the urban traffic cycle.
The most unfavorable engine toxic characteristics are the acceleration, deceleration and idling modes.
For the environmental assessment of automobile engines as a source of pollution, indicators are used that take into account the composition and quantity of exhaust gases, as well as the energy performance of vehicles.
The amount of component released by the engine per unit time (g/h) is calculated by the formula:
where Ci is the concentration of the toxic component in question, g/m3;
Qoi - volumetric exhaust gas flow rate, m3/h.
PROTECTION AGAINST NEGATIVE TECHNOGENIC IMPACTS OF A CAR
Car and traffic noise. The table shows sources of urban noise.
The transformation of energy in any machine, including a moving car, is associated with its dissipation in the surrounding space. One of the channels of such dispersion is sound waves. They represent the oscillatory movement of particles of an elastic medium, resulting from vibrations of the surface of the emitter or some aerodynamic process. The source of noise in a moving car is the surfaces of the engine power unit, the intake and exhaust systems, and the surfaces of transmission units. Noise also occurs when the car body interacts with the air flow during movement, the interaction of tires with the road surface, vibrations of suspension and body elements from road disturbances, etc. The table shows the distribution of the sound energy of a car from its various parts.
A person is able to perceive sound vibrations in the air in the frequency range from 20 to 20,000 Hz.
Transport noise is one of the most dangerous parameters of environmental pollution.
The space in which a sound wave exists and propagates is a sound field. Change physical condition environment in the sound field, caused by the presence of sound waves, is characterized by sound pressure (p), i.e., the difference between the value of the total pressure and the average pressure, which is usually observed in the air in the absence of sound waves. The unit of measurement for pressure is Pascal P=1 N/m2.
Sound vibrations are characterized by frequency f, which is determined through the speed of sound C and wavelength l. In isotropic media, the wavelength is related to the frequency and speed of sound by the relationship:
С=343.1 m/s at 200С.
The values of sound pressure, sound intensity and sound power vary over a very wide range. Thus, the sound pressure of the quietest sound that a person can perceive is 2 × 10-5 N/m2, and the upper limit can reach 2 × 104 N/m2. For such a wide range, it is appropriate to use relative units expressed in logarithmic units of decibels (dB). The unit of comparison for sound pressure is the threshold sound pressure equal to 2×10-5 N/m2.
Sound intensity level:
Where I0 is the threshold sound intensity at frequency f=1000 Hz, which corresponds to the threshold sound pressure p0=2×10-5 N/m2. A multiplier of 10 is used to obtain smaller units of noise - tenths of a bel.
The entire noise spectrum is divided into separate octaves. An octave is a frequency band in which the final frequency is 2 times greater than the initial one: fк=2 fн.
In occupational hygiene, it is customary to consider eight octaves with geometric mean frequencies of 63, 125, 250, 500, 1000, 2000, 4000 and 8000 Hz.
Car noise is broadband noise. To assess the impact of such noise on a person, frequency corrections are used, the characteristics of which are designated by the letters A, B, C. Characteristic A, corresponding to frequencies above 600 Hz, brings the noise measurement closer to human perception of sound.
The figure shows the dependence of the noise level on the speed of a passenger car in 1st gear (1), 2nd gear (2), 3rd gear (3) and 4th gear (4).
Impact of pollutants on flora, fauna and humans
from "Design of dust and gas purification devices"
The greatest damage to plants is caused by dispersed pollutants, metal compounds, fluorine, sulfur and nitrogen oxides. Dust and ash deposits on green mass limit the processes of photosynthesis, and metal compounds suppress them and act as cellular poisons. Fluoride compounds reduce forest productivity, causing trees to dry out and die. Sulfur and nitrogen oxides damage green mass and decompose chlorophyll. Coniferous trees are especially sensitive to them. Air pollution has a harmful effect on the flora and through the soil, where acid rain destroys soil bacteria, worms, decomposes humus, and washes away the elements necessary for plants.The influence of polluted atmosphere on animal world and humans are in many ways similar. Pollutants can cause intoxication, chronic and cancer diseases, increase the number of mutations, reduce reproduction and lifespan.
Numerous and, as a rule, scattered information on the animal world confirms that pollutants most often act specifically on different animal species, affecting certain organs and functions. One of the common manifestations of the consequences of air pollution is the lethality of acid rain for animals living in water bodies and soil. If the pH of water in reservoirs drops to 5, there is a massive death of fish, and water with a pH of less than 4.5 is generally unsuitable for animal life. In general, environmental pollution affects the animal world more noticeably than the human community. Every year the fauna of planet Earth becomes poorer by several more species.
Direct damage to human health is caused by fine airborne particles and gaseous pollutants. Dispersed particles with sizes less than 5 microns can reach the lungs without remaining in the nasopharynx and alveoli. Certain types of dust can cause specific diseases: silicate, coal, diamond and some others - pneumoconiosis, asbestos - cancer. Fine dust on which acids, acid-forming gases, toxic compounds and radionuclides are sorbed is very dangerous.
The degree of impact of pollutants on the human body depends on a huge number of reasons due to the state of the body itself, external conditions, the type of pollutant, and other factors. Very significant indicators are toxicity, concentration and exposure time of the pollutant. In general, it is generally accepted that long-term exposure to low concentrations is more dangerous than short-term exposure to concentrated substances, of course, if the dose received is not close to lethal.
At present, it is hardly possible to find a community of plants or animals on the planet that is not directly or indirectly influenced by humans.
The entire history of mankind is connected with one or another influence on flora and vegetation. At first it was a nomadic activity; After the advent of agriculture, the impact on the flora increased due to burning and deforestation to obtain new crop areas. Deforestation in the Mediterranean countries, Asia Minor, Mesopotamia (in regions of ancient cultural civilizations) led to significant xerophytization of vegetation and desertification of territories. In Africa, India, and South America, the reduction in tropical forest areas has led to an increase in the area of savannas. In Central Asia and North America, the increased development of livestock farming, in addition to desertification, caused the spread of shifting sand dunes. The era of the greats geographical discoveries led to the expansion of introduction and delivery of more and more new species, which often quickly spread in new conditions.
Usually we are talking about unconscious And conscious human impact on nature and its plant components (see Classification anthropogenic impacts- in the course of lectures on general ecology)
Unconscious influence(collecting plants, cutting down and burning forests) is usually beneficial to humans, but has a negative effect on vegetation. Unconscious selection by man has led to the creation of many cultivated plants, the history of which is largely unknown. Man often acts unconsciously and in the present, spreading seeds, spores and fruits due to powerful development various types transport.
Conscious influence can also be positive or negative. For example, artificial selection is an effective means of improving certain species and varieties or creating new breeds of cultivated plants. Reasonable deforestation within the limits of annual wood growth and subject to regeneration rules can significantly increase forest productivity; sanitary felling helps get rid of diseased trees. However, excess timber extraction in excess of annual growth, deforestation on mountain slopes, and destruction of vegetation in sanitary protection or water protection zones are examples of deliberate and negative influence.
Among the main directions of human influence on plants and vegetation, the following should be highlighted:
1. Enrichment of flora or change in the composition of vegetation. Until recently, unconscious enrichment of the flora prevailed in this direction. For example, whole line synanthropic plants have always accompanied humans during their settlement, but most of these species are classified as weeds and are of no practical value. Among them are:
Archaeophytes that have existed since prehistoric times (burdock, quinoa, cornflower, etc.);
Neophytes representing weed species of modern times (elodea - “water plague”, evening primrose, etc.);
Apophytes, local species that easily migrate to cultivated fields (flaxgrass, tuber-bearing china, sage, and yellow alfalfa often appear on freshly plowed fields).
Alien species associated with crops are called segetal (cornflower, brome), and weeds that prefer garbage dumps near houses or near roads are called ruderal (burdock, henbane, nettle).
Consciously cultivated in botanical gardens, parks or green areas, exotic foreign plants sometimes become native wildlife, e.g. acclimatize and naturalize. At the same time, they are forced to compete with local native species and withstand new climatic conditions. Only a few eurybiont species with a wide ecological amplitude and a large number of seeds can become part of the local flora. Examples: Impatiens parviflora from Central Asia and calamus (Acorus calamus) from Turkey have taken root in central Russia. Acclimatization and naturalization is easier in aquatic plants (for example, Elodea) due to the similarity of the ecological conditions of the aquatic environment.
2. Reduction of areas and habitats, as well as direct destruction of plants, occurs very often. Sometimes some species are deliberately destroyed: for example, in Scandinavia, barberry, which is an intermediate host of bread mark rust, is destroyed. Great damage to vegetation is caused by the uncontrolled collection of medicinal plants. Many plant species are classified as endangered and are included in the Red Books of various levels.
3. The direct impact of humans on vegetation is manifested by plowing land, deforestation, grazing of domestic animals, mowing and burning plants, especially in meadows and steppes.
4. Human influence on the flora is felt during various reclamation measures - during irrigation, watering or draining of individual territories. Irrigation is the artificial moistening of soils to obtain higher yields. It is known that the productivity of irrigated lands exceeds similar indicators of rain-fed lands several times. However, irrigation, especially excessive irrigation, in arid zones is associated with such a negative environmental phenomenon as secondary soil salinization. In the absence of drainage systems, areas of secondary saline soils are usually excluded from agricultural land use. In arid zones and deserts, people create special landscapes near water sources - oases - with a unique environmental situation. When watering, additional water sources are constructed (ponds, wells, boreholes), which are designed to improve water supply, but at the same time, irreversible pumping of water worsens the living conditions of plants. Land drainage is usually practiced in wetlands. As a rule, this increases the productivity of drained areas, but is accompanied by many negative side effects: a decrease in groundwater levels, shallowing and drying of rivers in neighboring non-wetland areas, etc.
5. Deterioration of the living conditions of plants also occurs as a result of air, water and soil pollution. Smoke, exposure to harmful gases, and acid rain are integral consequences of industrial activity.
6. The creation of littered (ruderal) habitats promotes the appearance of specific flora. Household waste dumps are characterized by a high nitrogen content due to the large amount of decomposing organic matter, therefore many plants growing in such dumps are nitrophilous. On the contrary, dumps and waste heaps contain virtually no soil humus, but discarded waste rocks are rich in a variety of toxic substances to which few plants can adapt. Significant areas occupied by such “monuments” of industrial production require various methods of reclamation and phytomelioration.
7. The most significant human impact on vegetation is due to the fact that artificial phytocenoses require everything more areas. However, the created agroecosystems are characterized by a depleted composition of vegetation due to the prevailing monoculture of agricultural production.
From time to time, oil tanker accidents occur around the world. A product spilled into the sea or ocean causes significant harm to the environment. Representatives of flora and fauna find themselves in a difficult situation.
Birds
The mechanism of the negative impact of petroleum products on birds has been studied in numerous studies. Oil spills cause the greatest harm to birds that spend most of their lives in water. Birds inhale fumes, drink water mixed with petroleum products, they remain on their feathers and enter the body during cleaning. Oil spills reduce bird numbers. A bird whose feathers are stained with petroleum products sits in its nest. Oil components get on the shell, which causes the death of the chick. After such accidents, the amount of fish and shellfish in the water decreases, which leads to a shortage of food for birds.
Research into the impact of oil spills on birds is ongoing. It has been proven that after such disasters, their species can recover provided they migrate to other areas not affected by the spill, if the individuals have not lost their ability to reproduce. It is possible to obtain accurate data on the negative impact of oil spills on birds, their death, and a decrease in the intensity of reproduction only at the sites of their colonies. It is more difficult to make an objective analysis when studying an entire species or to identify negative consequences on a regional scale.
Mammals
Oil spilled into the sea causes a lot of harm to mammals and causes their death. Petroleum products contaminate the fur, which clumps into clumps. Such a cover does not provide the animal with high-quality protection from water and cold. The oil corrodes the mucous membrane of the eyes, the mammal cannot navigate normally and get food. The penetration of petroleum products into the body causes bleeding of the gastrointestinal tract, liver poisoning with toxins, which leads to the death of the animal.
Reptiles and amphibians
The negative consequences of oil disasters among amphibians and reptiles have been poorly studied. Studies have shown that fresh oil has the greatest negative impact. Its exposure has a detrimental effect on the development of embryos, and cases of abnormal behavior have been observed.
Fish
Oil products spilled into the sea are sure to enter the body of fish living in this area with water and food. It has been noticed that after large-scale disasters, a large number of these representatives of marine fauna die. The intensity of the negative impact differs among different types. Studies of fish that died after the oil spill showed that the death was caused by an enlarged liver, poisoned by toxins. If oil enters the body of a fish, it disrupts the functioning of the heart; the product destroys the fins, making it impossible to swim. Negative changes have also been noted at the cellular level, leading to changes in behavior. Petroleum products have a detrimental effect on fish eggs and fry.
Invertebrates
Birds, fish, and animals have the opportunity to migrate in the event of oil disasters. This allows them to escape from negative consequences. Representatives of invertebrates do not have this opportunity. Therefore, they have to fully experience the harmful effects of petroleum products. The death of invertebrates is observed in the oil spill area not only immediately after the accident, but also for several years after the disaster. The duration of such a negative impact depends on the amount, type of oil, and other circumstances.
Plants
The most accurate are the results of studies of the impact on plants, since they are constantly in the disaster zone. Scientists have documented the extinction of sea grasses, algae and mangroves after oil accidents. Negative effects on representatives of the flora can last for five years. In small bodies of water the negative consequences are more serious than in the open sea. It has been proven that high-quality mechanical water purification carried out after a spill allows plants to recover 2 times faster in their previous quantities.
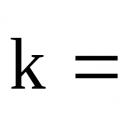 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe