The structure of the tellurium atom. Structure of the tellurium atom Tellurium: Dynamics of price changes on the world market
DEFINITION
Tellurium- fifty-second element of the Periodic Table. Designation - Te from the Latin "tellurium". Located in the fifth period, VIA group. Belongs to the metalloid family. The nuclear charge is 52.
Tellurium is one of the rare elements: its content is earth's crust is only 0.000001% (wt.).
In its free form, tellurium is a metal-like crystalline substance of silvery-white color (Fig. 1) with a hexagonal lattice. Brittle, easily abraded into powder. Semiconductor. Density 6.25 g/cm3. Melting point 450 o C, boiling point 990 o C.
It is known to exist in an amorphous state.
Rice. 1. Tellurium. Appearance.
Atomic and molecular mass of tellurium
The relative molecular mass of a substance (M r) is a number showing how many times the mass of a given molecule is greater than 1/12 the mass of a carbon atom, and the relative atomic mass of an element (A r) is how many times the average mass of atoms of a chemical element is greater than 1/12 mass of a carbon atom.
Since in the free state tellurium exists in the form of monatomic Te molecules, the values of its atomic and molecular masses coincide. They are equal to 127.60.
Isotopes of tellurium
It is known that in nature tellurium can be found in the form of eight stable isotopes, two of which are radioactive (128 Te and 130 Te): 120 Te, 122 Te, 123 Te, 124 Te, 125 Te and 126 Te. Their mass numbers are 120, 122, 123, 124, 125, 126, 128 and 130, respectively. The nucleus of an atom of the tellurium isotope 120 Te contains fifty-two protons and sixty-eight neutrons, and the remaining isotopes differ from it only in the number of neutrons.
There are artificial unstable isotopes of tellurium with mass numbers from 105 to 142, as well as eighteen isomeric states of nuclei.
Tellurium ions
At the outer energy level of the tellurium atom there are six electrons, which are valence:
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 4 .
As a result of chemical interaction, tellurium gives up its valence electrons, i.e. is their donor, and turns into a positively charged ion or accepts electrons from another atom, i.e. is their acceptor and turns into a negatively charged ion:
Te 0 -2e → Te + ;
Te 0 -4e → Te 4+ ;
Te 0 -6e → Te 6+ ;
Te 0 +2e → Te 2- .
Tellurium molecule and atom
In the free state, tellurium exists in the form of monatomic Te molecules. Here are some properties characterizing the tellurium atom and molecule:
Examples of problem solving
EXAMPLE 1
EXAMPLE 2
| Exercise | Calculate the mass fractions of the elements that make up tellurium dioxide if its molecular formula is TeO 2. |
| Solution | The mass fraction of an element in the composition of any molecule is determined by the formula: ω (X) = n × Ar (X) / Mr (HX) × 100%. |
It is unlikely that anyone will believe the story about the sea captain, who, in addition, is a professional circus wrestler, a famous metallurgist and a consultant physician at a surgical clinic. In the world of chemical elements, such a variety of professions is a very common phenomenon, and Kozma Prutkov’s expression does not apply to them: “A specialist is like gumboil: his completeness is one-sided.” Let us remember (even before talking about the main object of our story) iron in cars and iron in blood, iron is a concentrator magnetic field and iron - an integral part of ocher... True, the “professional training” of the elements sometimes took much more time than the training of an average qualified yogi. So element No. 52, which we are about to talk about, was used for many years only to demonstrate what it really is, this element named after our planet: “tellurium” - from tellus, which in Latin means “Earth” "
This element was discovered almost two centuries ago. In 1782, mining inspector Franz Joseph Müller (later Baron von Reichenstein) examined gold ore found in Semigorye, in what was then Austria-Hungary. It turned out to be so difficult to decipher the composition of the ore that it was called Aurum problematicum - “doubtful gold.” It was from this “gold” that Muller isolated a new metal, but there was no complete confidence that it was truly new. (It later turned out that Müller was wrong about something else: the element he discovered was new, but it can only be classified as a metal with great reserve.)
To dispel doubts, Müller turned for help to a prominent specialist, the Swedish mineralogist and analytical chemist Bergman.
Unfortunately, the scientist died before finishing the analysis of the sent substance - in those years, analytical methods were already quite accurate, but the analysis took a lot of time.
Other scientists also tried to study the element discovered by Müller, but only 16 years after its discovery, Martin Heinrich Klaproth, one of the leading chemists of that time, irrefutably proved that this element was in fact new and proposed the name “tellurium” for it.
As always, after the discovery of the element, the search for its applications began. Apparently, based on the old principle dating back to the times of atrochemistry - the world is a pharmacy, the Frenchman Fournier tried to treat some serious diseases with tellurium, in particular leprosy. But without success - only many years later was tellurium able to provide doctors with some “minor services”. More precisely, not tellurium itself, but salts of telluric acid K 2 Te0 3 and Na 2 Te0 3, which began to be used in microbiology as dyes that give a certain color to the bacteria being studied. Thus, with the help of tellurium compounds, the diphtheria bacillus is reliably isolated from a mass of bacteria. If not in treatment, then at least in diagnosis, element No. 52 turned out to be useful to doctors.
But sometimes this element, and even more so some of its compounds, add trouble to doctors. Tellurium is quite toxic. In our country, the maximum permissible concentration of tellurium in the air is 0.01 mg/m3. Of the tellurium compounds, the most dangerous is hydrogen telluride H 2 Te, a colorless poisonous gas with an unpleasant odor. The latter is quite natural: tellurium is an analogue of sulfur, which means that H 2 Te should be similar to hydrogen sulfide. It irritates the bronchi and has a harmful effect on the nervous system.
These unpleasant properties did not prevent tellurium from entering technology and acquiring many “professions.”
Metallurgists are interested in tellurium because even small additions to lead greatly increase the strength and chemical resistance of this important metal. Lead doped with tellurium is used in cable and chemical industry. Thus, the service life of sulfuric acid production devices coated on the inside with a lead-tellurium alloy (up to 0.5% Te) is twice as long as that of the same devices lined simply with lead. The addition of tellurium to copper and steel facilitates their machining.
In glass production, tellurium is used to give glass a brown color and a higher refractive index. In the rubber industry, it is sometimes used as an analogue of sulfur for the vulcanization of rubbers.
Tellurium - semiconductor
However, these industries were not responsible for the jump in prices and demand for element No. 52. This jump occurred in the early 60s of our century. Tellurium is a typical semiconductor, and a technological semiconductor. Unlike germanium and silicon, it melts relatively easily (melting point 449.8 ° C) and evaporates (boils at a temperature just below 1000 ° C). Consequently, it is easy to obtain thin semiconductor films from it, which are of particular interest to modern microelectronics.
However, pure tellurium as a semiconductor is used to a limited extent - for the manufacture of field-effect transistors of some types and in devices that measure the intensity of gamma radiation. Moreover, a tellurium impurity is deliberately introduced into gallium arsenide (the third most important semiconductor after silicon and germanium) in order to create electronic-type conductivity in it.
The scope of application of some tellurides - compounds of tellurium with metals - is much broader. Tellurides of bismuth Bi 2 Te 3 and antimony Sb 2 Te 3 have become the most important materials for thermoelectric generators. To explain why this happened, let's take a short digression into the field of physics and history.
A century and a half ago (in 1821), the German physicist Seebeck discovered that in a closed electrical circuit consisting of different materials, the contacts between which are at different temperatures, an electromotive force is created (it is called thermo-EMF). 12 years later, the Swiss Peltier discovered the effect, opposite effect Seebeck: when electricity flows through a circuit composed of different materials, at the contact points, in addition to the usual Joule heat, a certain amount of heat is released or absorbed (depending on the direction of the current).
For approximately 100 years, these discoveries remained “things in themselves”, curious facts, nothing more. And it would not be an exaggeration to say that new life Both of these effects began after Academician A.F. Ioffe and his colleagues developed the theory of using semiconductor materials for the manufacture of thermoelements. And soon this theory was embodied in real thermoelectric generators and thermoelectric refrigerators for various purposes.
In particular, thermoelectric generators, which use tellurides of bismuth, lead and antimony, provide energy to artificial Earth satellites, navigation and meteorological installations, and cathodic protection devices for main pipelines. The same materials help maintain the desired temperature in many electronic and microelectronic devices.
IN last years One more thing is of great interest chemical compound tellurium, which has semiconductor properties, is cadmium telluride CdTe. This material is used for the manufacture of solar cells, lasers, photoresistors, and radioactive radiation counters. Cadmium telluride is also famous for the fact that it is one of the few semiconductors in which the Han effect is noticeably manifested.
The essence of the latter is that the very introduction of a small plate of the corresponding semiconductor into a sufficiently strong electric field leads to the generation of high-frequency radio emission. The Hahn effect has already found application in radar technology.
In conclusion, we can say that quantitatively the main “profession” of tellurium is alloying lead and other metals. Qualitatively, the main thing, of course, is the work of tellurium and tellurides as semiconductors.
Useful admixture
In the periodic table, tellurium is located in the main subgroup of group VI next to sulfur and selenium. These three elements are similar in chemical properties and often accompany each other in nature. But the share of sulfur in the earth's crust is 0.03%, selenium is only 10-5%, tellurium is even an order of magnitude less - 10-6%. Naturally, tellurium, like selenium, is most often found in natural sulfur compounds - as an impurity. It happens, however (remember the mineral in which tellurium was discovered) that it comes into contact with gold, silver, copper and other elements. More than 110 deposits of forty tellurium minerals have been discovered on our planet. But it is always mined together with either selenium, or gold, or other metals.
In Russia, copper-nickel tellurium-containing ores of Pechenga and Monchegorsk, tellurium-containing lead-zinc ores of Altai and a number of other deposits are known.
Tellurium is isolated from copper ore at the stage of purifying blister copper by electrolysis. A sediment - sludge - falls to the bottom of the electrolyser. This is a very expensive intermediate product. To illustrate the composition of the sludge from one of the Canadian plants: 49.8% copper, 1.976% gold, 10.52% silver, 28.42% selenium and 3.83% tellurium. All these valuable components of the sludge must be separated, and there are several ways to do this. Here's one of them.
The sludge is melted in a furnace and air is passed through the melt. Metals, except gold and silver, oxidize and turn into slag. Selenium and tellurium are also oxidized, but into volatile oxides, which are captured in special devices (scrubbers), then dissolved and converted into acids - selenium H 2 SeO3 and telluric H 2 TeO3. If sulfur dioxide S0 2 is passed through this solution, reactions will occur
H 2 Se0 3 + 2S0 2 + H 2 0 → Se ↓ + 2H 2 S0 4 .
H2Te03 + 2S02 + H20 → Te ↓ + 2H 2 S0 4.
Tellurium and selenium fall out at the same time, which is highly undesirable - we need them separately. Therefore, the process conditions are selected in such a way that, in accordance with the laws of chemical thermodynamics, selenium is primarily reduced first. This is helped by selecting the optimal concentration of hydrochloric acid added to the solution.
Tellurium is then deposited. The resulting gray powder, of course, contains a certain amount of selenium and, in addition, sulfur, lead, copper, sodium, silicon, aluminum, iron, tin, antimony, bismuth, silver, magnesium, gold, arsenic, chlorine. Tellurium must first be purified from all these elements by chemical methods, then by distillation or zone melting. Naturally, tellurium is extracted from different ores in different ways.
Tellurium is harmful
Tellurium is being used more and more widely and, therefore, the number of people working with it is increasing. In the first part of the story about element No. 52, we already mentioned the toxicity of tellurium and its compounds. Let's talk about this in more detail - precisely because more and more people have to work with tellurium. Here is a quote from a dissertation on tellurium as an industrial poison: white rats injected with tellurium aerosol “showed restlessness, sneezed, rubbed their faces, and became lethargic and drowsy.” Tellurium has a similar effect on people.
And myself tellurium and its connections can bring troubles of different “calibers”. They, for example, cause baldness, affect blood composition, and can block various enzyme systems. Symptoms of chronic poisoning with elemental tellurium are nausea, drowsiness, emaciation; the exhaled air acquires a foul, garlicky odor of alkyl tellurides.
In case of acute tellurium poisoning, serum with glucose is administered intravenously, and sometimes even morphine. Ascorbic acid is used as a prophylactic. But the main prevention is the reliable sealing of devices, automation of processes in which tellurium and its compounds are involved.

Element No. 52 brings a lot of benefits and therefore deserves attention. But working with it requires caution, clarity and, again, concentrated attention.
APPEARANCE OF TELLURIUM. Crystalline tellurium is most similar to antimony. Its color is silver-white. Crystals are hexagonal, the atoms in them form spiral chains and are connected covalent bonds with the closest neighbors. Therefore, elemental tellurium can be considered an inorganic polymer. Crystalline tellurium is characterized by a metallic luster, although due to its complex of chemical properties it can rather be classified as a non-metal. Tellurium is brittle and quite easy to turn into powder. The question of the existence of an amorphous modification of tellurium has not been clearly resolved. When tellurium is reduced from telluric or telluric acid, a precipitate forms, but it is still not clear whether these particles are truly amorphous or just very small crystals.
BI-COLORED ANHYDRIDE. As befits an analogue of sulfur, tellurium exhibits valences of 2-, 4+ and 6+ and much less often 2+. Tellurium monoxide TeO can only exist in gaseous form and is easily oxidized to Te0 2. This is a white, non-hygroscopic, completely stable crystalline substance that melts without decomposition at 733 ° C; it has a polymer structure.
Tellurium dioxide is almost insoluble in water - only one part of Te0 2 per 1.5 million parts of water passes into the solution and a solution of weak telluric acid H 2 Te0 3 of negligible concentration is formed. Also weakly expressed acid properties and telluric acid
H 6 TeO 6 . This formula (and not H 2 TeO 4 was assigned to it after salts of the composition Ag 6 Te0 6 and Hg 3 Te0 6 were obtained, which are highly soluble in water. TeO3 anhydride, which forms telluric acid, is practically insoluble in water. This substance exists in two modifications - yellow and gray: α-TeO3 and β-TeO3. Gray tellurium anhydride is very stable: even when heated, it is not affected by acids and concentrated alkalis. It is purified from the yellow variety by boiling the mixture in concentrated caustic potassium.
SECOND EXCEPTION. When creating the periodic table, Mendeleev placed tellurium and its neighboring iodine (as well as argon and potassium) in groups VI and VII, not in accordance with, but contrary to them atomic weights. Indeed, the atomic mass of tellurium is 127.61, and that of iodine is 126.91. This means that iodine should not be behind tellurium, but in front of it. Mendeleev, however, did not doubt the right
the correctness of his reasoning, since he believed that the atomic weights of these elements were not determined accurately enough. Mendeleev's close friend, the Czech chemist Boguslav Brauner, carefully checked the atomic weights of tellurium and iodine, but his data coincided with the previous ones. The validity of exceptions confirming the rule was established only when the basis periodic table It was not the atomic weights that formed, but the nuclear charges, when the isotopic composition of both elements became known. Tellurium, unlike iodine, is dominated by heavy isotopes.
By the way, about isotones. There are currently 22 known isotopes of element No. 52. Eight of them - with mass numbers 120, 122, 123, 124, 125, 126, 128 and 130 - are stable. The last two isotopes are the most common: 31.79 and 34.48%, respectively.
TELLURIUM MINERALS. Although tellurium is significantly less abundant on Earth than selenium, more minerals of element No. 52 are known than those of its counterpart. Tellurium minerals are of two types in composition: either tellurides or products of the oxidation of tellurides in the earth's crust. Among the first are calaverite AuTe 2 and krennerite (Au, Ag) Te2, which are among the few natural gold compounds. Natural tellurides of bismuth, lead, and mercury are also known. Native tellurium is very rarely found in nature. Even before the discovery of this element, it was sometimes found in sulfide ores, but could not be correctly identified. Tellurium minerals have no practical significance - all industrial tellurium is a by-product of processing ores of other metals.
| Tellurium | |
|---|---|
| Atomic number | 52 |
| Appearance of a simple substance | |
| Properties of the atom | |
| Atomic mass (molar mass) |
127.6 a. e.m. (g/mol) |
| Atomic radius | 160 pm |
| Ionization energy (first electron) |
869.0 (9.01) kJ/mol (eV) |
| Electronic configuration | 4d 10 5s 2 5p 4 |
| Chemical properties | |
| Covalent radius | 136 pm |
| Ion radius | (+6e) 56,211 (-2e) pm |
| Electronegativity (according to Pauling) |
2,1 |
| Electrode potential | 0 |
| Oxidation states | +6, +4, +2 |
| Thermodynamic properties of a simple substance | |
| Density | 6.24 /cm³ |
| Molar heat capacity | 25.8 J/(mol) |
| Thermal conductivity | 14.3 W/(·) |
| Melting temperature | 722,7 |
| Heat of Melting | 17.91 kJ/mol |
| Boiling temperature | 1 263 |
| Heat of vaporization | 49.8 kJ/mol |
| Molar volume | 20.5 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | hexagonal |
| Lattice parameters | 4,450 |
| c/a ratio | 1,330 |
| Debye temperature | n/a |
Tellurium—a chemical element with atomic number 52 in the periodic table and atomic mass 127.60; denoted by the symbol Te (Tellurium), belongs to the metalloid family.
Story
It was first discovered in 1782 in the gold ores of Transylvania by mining inspector Franz Joseph Müller (later Baron von Reichenstein), on the territory of Austria-Hungary. In 1798, Martin Heinrich Klaproth isolated tellurium and determined its most important properties.
origin of name
From Latin tellus, Genitive telluris, Earth.
Being in nature
Native tellurium also occurs together with selenium and sulfur (Japanese telluric sulfur contains 0.17% Te and 0.06% Se).
An important source of tellurium is copper and lead ores.
Receipt
The main source is sludge from electrolytic refining of copper and lead. The sludge is fired, the tellurium remains in the cinder, which is washed with hydrochloric acid. Tellurium is isolated from the resulting hydrochloric acid solution by passing sulfur dioxide SO 2 through it.
To separate selenium and tellurium, add sulfuric acid. In this case, tellurium dioxide TeO 2 falls out, and H 2 SeO 3 remains in solution.
Tellurium is reduced from TeO2 oxide with coal.
To purify tellurium from sulfur and selenium, its ability, under the action of a reducing agent (Al) in an alkaline medium, to transform into soluble disodium ditelluride Na 2 Te 2 is used:
6Te + 2Al + 8NaOH = 3Na 2 Te 2 + 2Na.
To precipitate tellurium, air or oxygen is passed through the solution:
2Na 2 Te 2 + 2H 2 O + O 2 = 4Te + 4NaOH.
To obtain tellurium of special purity, it is chlorinated
Te + 2Cl 2 = TeCl 4.
The resulting tetrachloride is purified by distillation or rectification. The tetrachloride is then hydrolyzed with water:
TeCl 4 + 2H 2 O = TeO 2 + 4HCl,
and the resulting TeO 2 is reduced with hydrogen:
TeO 2 + 4H 2 = Te + 2H 2 O.
Prices
Tellurium is a rare element, and significant demand with a small volume of production determines its high price (about 200-300 dollars per kg, depending on purity), but despite this, the range of areas for its application is constantly expanding.
Physicochemical characteristics
Tellurium is a brittle, silvery-white substance with a metallic luster. In thin layers, when exposed to light, it is red-brown, in pairs it is golden-yellow.
Chemically, tellurium is less active than sulfur. It dissolves in alkalis, is susceptible to the action of nitric and sulfuric acids, but in dilute hydrochloric acid dissolves slightly. Tellurium metal begins to react with water at 100°C, and in powder form it oxidizes in air even at room temperature, forming Te0 2 oxide.
When heated in air, tellurium burns, forming Te0 2. This strong compound is less volatile than tellurium itself. Therefore, to purify tellurium from oxides, they are reduced with flowing hydrogen at 500-600 °C.
In the molten state, tellurium is quite inert, so graphite and quartz are used as container materials when melting it.
Application
Alloys
Tellurium is used in the production of lead alloys with increased ductility and strength (used, for example, in the production of cables). With the introduction of 0.05% tellurium, the loss of lead due to dissolution under the influence of sulfuric acid is reduced by 10 times, and this is used in the production of lead-acid batteries. It is also important that lead doped with tellurium does not soften when processed by plastic deformation, and this makes it possible to use the technology for manufacturing battery plate current collectors using the cold cutting method and significantly increase the service life and specific characteristics of the battery.
Thermoelectric materials
Bismuth telluride single crystal
Its role is also great in the production of semiconductor materials and, in particular, tellurides of lead, bismuth, antimony, and cesium. In the coming years, the production of lanthanide tellurides, their alloys and alloys with metal selenides will become very important for the production of thermoelectric generators with very high (up to 72-78%) efficiency, which will make it possible to use them in the energy sector and in the automotive industry.
For example, a very high thermal emf was recently discovered in manganese telluride (500 μV/K) and in its combination with selenides of bismuth, antimony and lanthanides, which makes it possible not only to achieve very high efficiency in thermogenerators, but also to implement it already in one stage of semiconductor refrigerator cooling down to the cryogenic (temperature level of liquid nitrogen) temperatures and even lower. The best tellurium-based material for the production of semiconductor refrigerators in recent years has been tellurium alloy,
It is unlikely that anyone will believe the story about the sea captain, who, in addition, is a professional circus wrestler, a famous metallurgist and a consultant physician at a surgical clinic. In the world of chemical elements, such a variety of professions is a very common phenomenon, and Kozma Prutkov’s expression does not apply to them: “A specialist is like gumboil: his completeness is one-sided.” Let us remember (even before talking about the main object of our story) iron in cars and iron in blood, iron is a magnetic field concentrator and iron is an integral part of ocher... True, the “professional training” of elements sometimes took much more time than preparation intermediate yoga. So element No. 52, which we are about to talk about, was used for many years only to demonstrate what it really is, this element named after our planet: “tellurium” - from tellus, which in Latin means “Earth”. "
This element was discovered almost two centuries ago. In 1782, mining inspector Franz Joseph Müller (later Baron von Reichenstein) examined gold ore found in Semigorye, in what was then Austria-Hungary. It turned out to be so difficult to decipher the composition of the ore that it was called Aurum problematicum - “doubtful gold.” It was from this “gold” that Muller isolated a new metal, but there was no complete confidence that it was truly new. (It later turned out that Müller was wrong about something else: the element he discovered was new, but it can only be classified as a metal with great reserve.)
To dispel doubts, Müller turned for help to a prominent specialist, the Swedish mineralogist and analytical chemist Bergman.
Unfortunately, the scientist died before finishing the analysis of the sent substance - in those years, analytical methods were already quite accurate, but the analysis took a lot of time.
Other scientists also tried to study the element discovered by Müller, but only 16 years after its discovery, Martin Heinrich Klaproth, one of the leading chemists of that time, irrefutably proved that this element was actually new and proposed the name “tellurium” for it.
As always, after the discovery of the element, the search for its applications began. Apparently, based on the old principle dating back to the times of atrochemistry - the world is a pharmacy, the Frenchman Fournier tried to treat some serious diseases with tellurium, in particular leprosy. But without success - only many years later was tellurium able to provide doctors with some “minor services”. More precisely, not tellurium itself, but salts of telluric acid K 2 TeO 3 and Na 2 TeO 3, which began to be used in microbiology as dyes that give a certain color to the bacteria being studied. Thus, with the help of tellurium compounds, the diphtheria bacillus is reliably isolated from a mass of bacteria. If not in treatment, then at least in diagnosis, element No. 52 turned out to be useful to doctors.
But sometimes this element, and even more so some of its compounds, add trouble to doctors. Tellurium is quite toxic. In our country, the maximum permissible concentration of tellurium in the air is considered to be 0.01 mg/m3. Of the tellurium compounds, the most dangerous is hydrogen telluride H 2 Te, a colorless poisonous gas with an unpleasant odor. The latter is quite natural: tellurium is an analogue of sulfur, which means that H 2 Te should be similar to hydrogen sulfide. It irritates the bronchi and has a harmful effect on the nervous system.
These unpleasant properties did not prevent tellurium from entering technology and acquiring many “professions.”
Metallurgists are interested in tellurium because even small additions to lead greatly increase the strength and chemical resistance of this important metal. Lead doped with tellurium is used in the cable and chemical industries. Thus, the service life of sulfuric acid production devices coated on the inside with a lead-tellurium alloy (up to 0.5% Te) is twice as long as that of the same devices lined simply with lead. The addition of tellurium to copper and steel facilitates their machining.
In glass production, tellurium is used to give glass a brown color and a higher refractive index. In the rubber industry, it is sometimes used as an analogue of sulfur for the vulcanization of rubbers.
Tellurium is a semiconductor
However, these industries were not responsible for the jump in prices and demand for element No. 52. This leap occurred in the early 60s of our century. Tellurium is a typical semiconductor, and a technological semiconductor. Unlike germanium and silicon, it melts relatively easily (melting point 449.8°C) and evaporates (boils at just below 1000°C). Consequently, it is easy to obtain thin semiconductor films from it, which are of particular interest to modern microelectronics.
However, pure tellurium as a semiconductor is used to a limited extent - for the manufacture of field-effect transistors of some types and in devices that measure the intensity of gamma radiation. Moreover, a tellurium impurity is deliberately introduced into gallium arsenide (the third most important semiconductor after silicon and germanium) in order to create electronic-type conductivity in it*.
* The two types of conductivity inherent in semiconductors are described in detail in the article “Germanium”.
The scope of application of some tellurides - compounds of tellurium with metals - is much broader. Tellurides of bismuth Bi 2 Te 3 and antimony Sb 2 Te 3 have become the most important materials for thermoelectric generators. To explain why this happened, let's take a short digression into the field of physics and history.
A century and a half ago (in 1821), the German physicist Seebeck discovered that in a closed electrical circuit consisting of different materials, the contacts between which are at different temperatures, an electromotive force is created (it is called thermo-EMF). After 12 years, the Swiss Peltier discovered an effect opposite to the Seebeck effect: when an electric current flows through a circuit composed of different materials, at the contact points, in addition to the usual Joule heat, a certain amount of heat is released or absorbed (depending on the direction of the current).
For approximately 100 years, these discoveries remained “things in themselves”, curious facts, nothing more. And it would not be an exaggeration to say that a new life for both of these effects began after the Hero of Socialist Labor, Academician A.F. Ioffe and his colleagues developed a theory of using semiconductor materials for the manufacture of thermoelements. And soon this theory was embodied in real thermoelectric generators and thermoelectric refrigerators for various purposes.
In particular, thermoelectric generators, which use tellurides of bismuth, lead and antimony, provide energy to artificial Earth satellites, navigation and meteorological installations, and cathodic protection devices for main pipelines. The same materials help maintain the desired temperature in many electronic and microelectronic devices.
In recent years, another tellurium chemical compound with semiconductor properties, cadmium telluride CdTe, has attracted great interest. This material is used for the manufacture of solar cells, lasers, photoresistors, and radiation counters. Cadmium telluride is also famous for the fact that it is one of the few semiconductors in which the Han effect is noticeably manifested.
The essence of the latter is that the very introduction of a small plate of the corresponding semiconductor into a sufficiently strong electric field leads to the generation of high-frequency radio emission. The Hahn effect has already found application in radar technology.
In conclusion, we can say that quantitatively the main “profession” of tellurium is alloying lead and other metals. Qualitatively, the main thing, of course, is the work of tellurium and tellurides as semiconductors.
Useful admixture
In the periodic table, tellurium is located in the main subgroup of group VI next to sulfur and selenium. These three elements are similar in chemical properties and often accompany each other in nature. But the share of sulfur in the earth’s crust is 0.03%, selenium is only 10–5%, and tellurium is even an order of magnitude less – 10–6%. Naturally, tellurium, like selenium, is most often found in natural sulfur compounds - as an impurity. It happens, however (remember the mineral in which tellurium was discovered) that it comes into contact with gold, silver, copper and other elements. More than 110 deposits of forty tellurium minerals have been discovered on our planet. But it is always mined together with either selenium, or gold, or other metals.
In the USSR, copper-nickel tellurium-containing ores of Pechenga and Monchegorsk, tellurium-containing lead-zinc ores of Altai and a number of other deposits are known.
Tellurium is isolated from copper ore at the stage of purifying blister copper by electrolysis. A sediment - sludge - falls at the bottom of the electrolyser. This is a very expensive intermediate product. To illustrate the composition of the sludge from one of the Canadian plants: 49.8% copper, 1.976% gold, 10.52% silver, 28.42% selenium and 3.83% tellurium. All these valuable components of the sludge must be separated, and there are several ways to do this. Here's one of them.
The sludge is melted in a furnace and air is passed through the melt. Metals, except gold and silver, oxidize and turn into slag. Selenium and tellurium are also oxidized, but into volatile oxides, which are captured in special devices (scrubbers), then dissolved and converted into acids - selenium H 2 SeO 3 and telluric H 2 TeO 3 . If sulfur dioxide SO2 is passed through this solution, the following reactions will occur:
H 2 SeO 3 + 2SO 2 + H 2 O → Se ↓ + 2H 2 SO 4,
H 2 TeO 3 + 2SO 2 + H 2 O → Te ↓ + 2H 2 SO 4.
Tellurium and selenium fall out at the same time, which is very undesirable - we need them separately. Therefore, the process conditions are selected in such a way that, in accordance with the laws of chemical thermodynamics, selenium is primarily reduced first. This is helped by selecting the optimal concentration of hydrochloric acid added to the solution.
Tellurium is then deposited. The resulting gray powder, of course, contains a certain amount of selenium and, in addition, sulfur, lead, copper, sodium, silicon, aluminum, iron, tin, antimony, bismuth, silver, magnesium, gold, arsenic, chlorine. Tellurium must first be purified from all these elements by chemical methods, then by distillation or zone melting. Naturally, tellurium is extracted from different ores in different ways.
Tellurium is harmful
Tellurium is being used more and more widely and, therefore, the number of people working with it is increasing. In the first part of the story about element No. 52, we already mentioned the toxicity of tellurium and its compounds. Let’s talk about this in more detail, precisely because more and more people have to work with tellurium. Here is a quote from a dissertation on tellurium as an industrial poison: white rats injected with tellurium aerosol “showed restlessness, sneezed, rubbed their faces, and became lethargic and drowsy.” Tellurium has a similar effect on people.
And tellurium itself and its compounds can bring troubles of different “calibers”. They, for example, cause baldness, affect blood composition, and can block various enzyme systems. Symptoms of chronic poisoning with elemental tellurium are nausea, drowsiness, emaciation; the exhaled air acquires a foul, garlicky odor of alkyl tellurides.
In case of acute tellurium poisoning, serum with glucose, and sometimes even morphine, is administered intravenously. Ascorbic acid is used as a prophylactic. But the main prevention is case sealing of devices, automation of processes in which tellurium and its compounds are involved.
Element No. 52 brings a lot of benefits and therefore deserves attention. But working with it requires caution, clarity and, again, concentrated attention.
Appearance of tellurium
Crystalline tellurium is most similar to antimony. Its color is silvery-white. Crystals are hexagonal, the atoms in them form helical chains and are connected by covalent bonds to their nearest neighbors. Therefore, elemental tellurium can be considered an inorganic polymer. Crystalline tellurium is characterized by a metallic luster, although due to its complex of chemical properties it can rather be classified as a non-metal. Tellurium is brittle and quite easy to turn into powder. The question of the existence of an amorphous modification of tellurium has not been clearly resolved. When tellurium is reduced from telluric or telluric acid, a precipitate forms, but it is still not clear whether these particles are truly amorphous or just very small crystals.
Bicolor anhydride
As befits a sulfur analogue, tellurium exhibits valences of 2–, 4+ and 6+, and much less often 2+. Tellurium monoxide TeO can only exist in gaseous form and is easily oxidized to TeO 2 . This is a white, non-hygroscopic, completely stable crystalline substance that melts without decomposition at 733°C; it has a polymer structure, the molecules of which are built like this:
Tellurium dioxide is almost insoluble in water - only one part of TeO 2 per 1.5 million parts of water passes into the solution and a solution of weak telluric acid H 2 TeO 3 of negligible concentration is formed. The acidic properties of telluric acid H 6 TeO 6 are also weakly expressed. This formula (and not H 2 TeO 4) was assigned to it after salts of the composition Ag 6 TeO 6 and Hg 3 TeO 6, which are highly soluble in water, were obtained. The anhydride TeO 3 that forms telluric acid is practically insoluble in water. This substance exists in two modifications - yellow and gray: α-TeO 3 and β-TeO 3. Gray tellurium anhydride is very stable: even when heated, it is not affected by acids and concentrated alkalis. It is purified from the yellow variety by boiling the mixture in concentrated caustic potassium.
Second exception
When creating the periodic table, Mendeleev placed tellurium and its neighboring iodine (as well as argon and potassium) in groups VI and VII not in accordance with, but contrary to their atomic weights. Indeed, the atomic mass of tellurium is 127.61, and that of iodine is 126.91. This means that iodine should not be behind tellurium, but in front of it. Mendeleev, however, did not doubt the correctness of his reasoning, since he believed that the atomic weights of these elements were not determined accurately enough. Mendeleev's close friend, the Czech chemist Boguslav Brauner, carefully checked the atomic weights of tellurium and iodine, but his data coincided with the previous ones. The validity of exceptions confirming the rule was established only when the periodic system was based not on atomic weights, but on nuclear charges, when the isotopic composition of both elements became known. Tellurium, unlike iodine, is dominated by heavy isotopes.
By the way, about isotopes. Currently, 22 isotopes of element No. 52 are known. Eight of them - with mass numbers 120, 122, 123, 124, 125, 126, 128 and 130 - are stable. The last two isotopes are the most common: 31.79 and 34.48%, respectively.
Tellurium minerals
Although tellurium is significantly less abundant on Earth than selenium, more minerals of element 52 are known than those of its counterpart. Tellurium minerals are of two types in composition: either tellurides or products of the oxidation of tellurides in the earth's crust. Among the first are calaverite AuTe 2 and krennerite (Au, Ag) Te 2, which are among the few natural gold compounds. Natural tellurides of bismuth, lead, and mercury are also known. Native tellurium is very rarely found in nature. Even before the discovery of this element, it was sometimes found in sulfide ores, but could not be correctly identified. Tellurium minerals have no practical significance - all industrial tellurium is a by-product of processing ores of other metals.
Discovered by F. Müller in 1782. The name of the element comes from the Latin tellus, genitive telluris, Earth (the name was proposed by M. G. Klaproth, who isolated the element as a simple substance and determined its most important properties).
Receipt:
In nature, it exists as a mixture of 8 stable isotopes (120, 122-126, 128, 130). The content in the earth's crust is 10 -7%. The main minerals are altaite (PbTe), tellurobismuthite (Bi 2 Te 3), tetradymite (Bi 2 Te 2 S), found in many sulfide ores.
It is obtained from copper production sludge by leaching with a NaOH solution in the form of Na 2 TeO 3 , from which tellurium is separated electrolytically. Further purification is by sublimation and zone melting.
Physical properties:
Compact tellurium is a silvery-gray substance with a metallic luster, having a hexagonal crystal lattice (density 6.24 g/cm 3, melting point - 450°C, boiling point - 990°C). From solutions it precipitates in the form of a brown powder; in vapor it consists of Te 2 molecules.
Chemical properties:
Tellurium is stable in air at room temperature; when heated, it reacts with oxygen. Interacts with halogens and reacts with many metals when heated.
When heated, tellurium is oxidized by water vapor to form tellurium(II) oxide and reacts with concentrated sulfuric and nitric acids. When boiled in aqueous solutions of alkalis, it disproportions similarly to sulfur:
8 Te + 6NaOH = Na 2 TeO 3 + 2Na 2 Te + 3H 2 O
In compounds it exhibits oxidation states -2, +4, +6, less often +2.
The most important connections:
Tellurium(IV) oxide Tellurium dioxide, TeO 2, is poorly soluble in water, an acidic oxide, reacts with alkalis to form telluric acid salts. Used in laser technology, a component of optical glasses.
Tellurium(VI) oxide, tellurium trioxide, TeO 3, yellow or gray substance, practically insoluble in water, decomposes when heated to form dioxide, reacts with alkalis. Obtained by the decomposition of telluric acid.
Telluric acid, H 2 TeO 3 , slightly soluble, prone to polymerization, therefore it usually represents a precipitate with variable water content TeO 2 *nH 2 O. Salts - tellurites(M 2 TeO 3) and polytellurites (M 2 Te 2 O 5, etc.), usually obtained by sintering carbonates with TeO 2, are used as components of optical glasses.
Telluric acid, H 6 TeO 6 , white crystals, highly soluble in hot water. A very weak acid, in solution it forms salts of the composition MH 5 TeO 6 and M 2 H 4 TeO 6. When heated in a sealed ampoule, metatelluric acid H 2 TeO 4 was also obtained, which in solution gradually turns into telluric acid. Salts - tellurates. It is also obtained by fusing tellurium(IV) oxide with alkalis in the presence of oxidizing agents, or by fusing telluric acid with carbonate or metal oxide. Tellurates alkali metals soluble. They are used as ferroelectrics, ion exchangers, and components of luminescent compositions.
Hydrogen telluride, H 2 Te is a poisonous gas with an unpleasant odor, obtained by hydrolysis of aluminum telluride. A strong reducing agent, in solution it is quickly oxidized by oxygen to tellurium. In an aqueous solution, the acid is stronger than sulfur and hydrogen selenide. Salts - tellurides, are usually obtained by interaction simple substances, alkali metal tellurides are soluble. Many p- and d-element tellurides are semiconductors.
Halides. Tellurium(II) halides, for example TeCl 2 , are known to be salt-like and, when heated and in solution, disproportionate into Te and Te(IV) compounds. Tellurium tetrahalides - solids, hydrolyze in solution to form telluric acid, easily forming complex halides (for example K 2 ). TeF 6 hexafluoride, a colorless gas, unlike sulfur hexafluoride, is easily hydrolyzed, forming telluric acid.
Application:
Component of semiconductor materials; alloying additive for cast iron, steel, lead alloys.
World production (without the USSR) is about 216 tons/year (1976).
Tellurium and its compounds are toxic. MPC is about 0.01 mg/m3.
See also: Tellurium // Wikipedia. (access date: 12/23/2019).
"Discovery of the elements and the origin of their names."
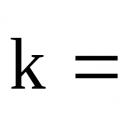 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe