Forms the basis of the internal environment of living organisms. Inorganic substances
Water – the most common substance. Seas and oceans occupy 71% of the globe's surface. However, recently there has been a shortage of fresh water, because... Salt water is used little by people, and fresh water is used for irrigation and industry.
Density. In water, the weight of all organisms is lighter, and many organisms float in the water without sinking to the bottom. But the density of water makes movement difficult, so organisms must have well-developed muscles to swim quickly. With depth, pressure increases greatly - deep-sea inhabitants endure pressure.
Light. Penetrates to a shallow depth. Therefore, plants exist only in the upper horizons. At great depths, animals live in complete darkness.
Temperature conditions. Temperature fluctuations in the water are smoothed out; aquatic inhabitants do not adapt to severe frost and heat.
Limited amount of oxygen. Its solubility is not very high and decreases with contamination or heating. Therefore, there are death in reservoirs due to lack of oxygen.
Salt composition.
The polarity of molecules and the ability to form hydrogen bonds make water a good solvent for a huge number of inorganic and organic substances. Most chemical reactions involve interactions between water-soluble substances. Under the action of enzymes, water enters into hydrolysis reactions, in which OH - and H + water are added to the free valences of various molecules. Water forms the basis of the internal environment of living organisms. Water ensures the influx of substances into the cell and their removal through the outer cell membrane (transport function). Water is a heat regulator. Due to the good thermal conductivity and greater heat capacity of water, when the temperature of the environment changes, inside the cell t remains unchanged or its fluctuations are much less than in the environment. Water is a donor of electrons and protons in energy metabolism. Water participates in the formation of higher structures of biological macromolecules. Cellular metabolism depends on the balance of free and bound water. Water has a high heat capacity. The specific heat capacity of water is the amount of heat required to raise the temperature of 1 kg of water by 1 0. Water is the only substance that has a higher density in the liquid state than in the solid state. There is surface tension on the surface of water.
Water- a complex living system where plants, animals and microorganisms live, which constantly multiply and die, which ensures the self-purification of water bodies.
Water has the greatest density at t 4 0 C (1 g/cm 3), so in winter water bodies do not freeze. Water molecules have polarity and are attracted to each other by opposite poles, forming associations due to hydrogen bonds. Doubled water molecules, which have 2 hydrogen bonds, are the most stable. Water molecules are resistant to heating; only at t 1000 0 C does steam begin to dissociate into H and O 2. Compound natural water. 5 groups of substances: 1. main ions (cations: Na +, Ca 2+, Mg 2+, Mn 2+, Fe 2+, Fe 3+, K +), 2. anions (HCO 3-, SO 4 2- , Cl - , CO 3 2- , SO 3 2- , S 2 O 3-), 3. dissolved gases (CO 2 O 2 N 2 H 2 S CH 4), 4. nutrients (NH 3 - ammonia, nitrites , nitrates, P, Si), 5. microelements (I, F, Cu, Br, CO, Ni). Natural waters are divided into carbonate, hydrocarbonate, sulfate, and chloride based on the content of anions. According to the content of cations: calcium, magnesium and sodium water. The salt content in water affects the corrosion of metal, concrete and stone materials. Mineralization of river water is 200-1000 mg/l, lake water is 15-300 mg/l, sea water is 3500 mg/l. Indicators of the entry of organic substances into water are chlorides, ammonia and nitrates. Water pollution with organic matter is accompanied by an increase in anaerobic and aerobic bacteria and fungi. Ammonia (MPC – 2 mg/l) indicates fresh water contamination. In deep underground waters, the presence of ammonia is possible, which is formed due to the reduction of nitrates in the absence of O 2. In swampy and peat waters, the ammonia content is not an indicator of pollution (ammonia of plant origin). Nitrites (KNO 2, HNO 2) are products of ammonia oxidation during the nitrification process, indicating the age of contamination. Nitrates (MPC – 10 mg/l) are the final product of mineralization. If ammonia, nitrates and nitrites are present at the same time, the water is dangerous in terms of epidemics. Nitrates (Ca(NO 3) 2, NaNO 3, KNO 3) can be contained due to the dissolution of soil salts, mineral fertilizers, and nitrate. Nitrates are precursors for the formation of carcinogenic substances - nitrosamines. They reduce the body's resistance to the effects of mutagenic and carcinogenic factors. Chlorides are an indicator of household pollution (MPC – 20-30 mg/l). In places with saline soil, chlorides of salt origin are present in groundwater. Wells and drainages should not be contaminated with organic substances. They should be located on uncontaminated elevated areas, at least 50 m away from latrines, cesspools, sewer networks, livestock yards, cemeteries, fertilizer and pesticide storage facilities.
Life forms of hydrobionts. In the water column (pelagial): 1. plankton – organisms (algae, protozoa, crustaceans) incapable of active movement and unable to withstand water currents. Cryoplankton (flagellates) - the population of melt water, is formed under the rays of the sun in ice cracks and snow voids. 2. nekton - large animals whose motor activity is sufficient to overcome water currents (fish, squid, mammals). 3. pleiston – organisms, part of whose body is in the water, and part above the surface (duckweed, gastropods, fish). 4. benthos (bacteria, actinomycetes, algae and fungi, protozoa, sponges, corals, annelids, crustaceans, echinoderms, insect larvae) live on the surface of the soil (epibenthos) and in its thickness (endobenthos). In the zone of contact of the water column with the bottom there is pelagobenthos. 5. periphyton – fouling organisms – all organisms living on dense substrates outside the bottom layer of water (bivalves and barnacles, sponges). 6. neuston – organisms living in the surface layer of water. On the surface of the water film there is epineuston (water strider bugs, flies) or under it there is hyponeuston (copepods, juvenile fish, insects, mollusk larvae).
The phrase “internal environment of the body” appeared thanks to a French physiologist who lived in the 19th century. In his works, he emphasized that a necessary condition for the life of an organism is to maintain constancy in the internal environment. This position became the basis for the theory of homeostasis, which was formulated later (in 1929) by the scientist Walter Cannon.
Homeostasis is the relative dynamic constancy of the internal environment,
As well as some static physiological functions. The internal environment of the body is formed by two fluids - intracellular and extracellular. The fact is that each cell of a living organism performs a specific function, so it needs a constant supply of nutrients and oxygen. She also feels the need to constantly remove waste products. The necessary components can penetrate the membrane only in a dissolved state, which is why each cell is washed by tissue fluid, which contains everything necessary for its life. It belongs to the so-called extracellular fluid, and accounts for 20 percent of body weight.
The internal environment of the body, consisting of extracellular fluid, contains:
- lymph (component of tissue fluid) - 2 l;
- blood - 3 l;
- interstitial fluid - 10 l;
- transcellular fluid - about 1 liter (it includes cerebrospinal, pleural, synovial, intraocular fluids).
They all have different compositions and differ in their functional

Properties. Moreover, the internal environment may have a small difference between the consumption of substances and their intake. Because of this, their concentration constantly fluctuates. For example, the amount of sugar in the blood of an adult can range from 0.8 to 1.2 g/l. If the blood contains more or less of certain components than necessary, this indicates the presence of a disease.
As already noted, the internal environment of the body contains blood as one of its components. It consists of plasma, water, proteins, fats, glucose, urea and mineral salts. Its main location is (capillaries, veins, arteries). Blood is formed due to the absorption of proteins, carbohydrates, fats, and water. Its main function is the relationship of organs with the external environment, delivery of necessary substances to organs, and removal of decay products from the body. It also performs protective and humoral functions.

Tissue fluid consists of water and nutrients dissolved in it, CO 2, O 2, as well as dissimilation products. It is located in the spaces between tissue cells and is formed due to Tissue fluid is intermediate between blood and cells. It transfers O2, mineral salts,
Lymph consists of water and dissolved in it. It is located in the lymphatic system, which consists of vessels merged into two ducts and flowing into the vena cava. It is formed by tissue fluid, in sacs that are located at the ends of lymphatic capillaries. The main function of lymph is to return tissue fluid to the bloodstream. In addition, it filters and disinfects tissue fluid.
As we see, the internal environment of the body is a set of physiological, physico-chemical, respectively, and genetic conditions that affect the viability of a living being.
2014-05-31Among the inorganic compounds of living organisms, water plays a special role. Water is the main medium in which metabolic processes and energy conversion occur.
The water content in most living organisms is 60-70%. Water forms the basis of the internal environment of living organisms (blood, lymph, intercellular fluid). The unique properties of water are determined by the structure of its molecules. In a water molecule, one oxygen atom is covalently bonded to two hydrogen atoms. The water molecule is polar (dipole). The positive charge is concentrated on the hydrogen atoms because oxygen is more electronegative than hydrogen. The negatively charged oxygen atom of one water molecule is attracted to the positively charged hydrogen atom of another molecule, thereby forming a hydrogen bond, which is 15-20 times weaker than a covalent bond. Therefore, hydrogen bonds are easily broken, which is observed, for example, during the evaporation of water. Due to the thermal movement of molecules in water, some hydrogen bonds are broken and some are formed.
Thus, the molecules are mobile in a liquid state, which is very important for metabolic processes. Water molecules easily penetrate cell membranes. Due to the high polarity of its molecules, water is a solvent for other polar compounds. Depending on the ability of certain compounds to dissolve in water, they are conventionally divided into hydrophilic, or polar, and hydrophobic, or non-polar. Most salts are hydrophilic compounds that are soluble in water. Hydrophobic compounds (almost all fats, some proteins) contain non-polar groups and do not form hydrogen bonds, so these compounds are not soluble in water. It has a high heat capacity and at the same time high thermal conductivity for liquids. These properties make water ideal for maintaining thermal balance in the body.
To maintain the vital processes of individual cells and the body as a whole, mineral salts are important. Living organisms contain both dissolved salts (in the form of ions) and salts in the solid state. Ions are divided into positive (cations of metal elements K +, Na +, Ca2 +, M2 +, etc.) and negative (anions of hydrochloric acids - Cl -, sulfuric acids - HSO4 -, SO42 -, carbonate acids - HCO3 -, phosphate acids - H2PO4 - , HPO42 - etc.).. Different concentrations of K + and Na + cations in the cell and intercellular fluid cause a potential difference on the cell membrane; a change in membrane permeability to K + and Na + under the influence of irritation ensures the occurrence of nervous and muscle excitation. Phosphoric acid anions support the neutral reaction of the intracellular environment (pH = 6.9), carboxylic acid anions support the slightly alkaline reaction of the blood plasma (pH = 7.4). Calcium compounds (CaCO3) are part of the shells of mollusks and protozoa, and the shells of crayfish. Hydrochloric acid creates an acidic environment in the stomach of vertebrates and humans, thereby ensuring the activity of gastric juice enzymes. Residues of sulfuric acid join water-insoluble compounds, ensuring their solubility, which contributes to the removal of these compounds from cells and the body.
CELL BIOLOGY
Inorganic substances
Among the inorganic compounds of living organisms, water plays a special role. Water is the main medium in which metabolic processes and energy conversion occur. The water content in most living organisms is 60-70%. Water forms the basis of the internal environment of living organisms (blood, lymph, intercellular fluid). The unique properties of water are determined by the structure of its molecules. In a water molecule, one Oxygen atom is covalently bonded to two Hydrogen atoms. The water molecule is polar (dipole). The positive charge is concentrated on the Hydrogen atoms because Oxygen is more electronegative than Hydrogen. The negatively charged Oxygen atom of one water molecule is attracted to the positively charged Hydrogen atom of another molecule, thereby forming a hydrogen bond, which is 15-20 times weaker than a covalent bond. Therefore, hydrogen bonds are easily broken, which is observed, for example, during the evaporation of water. Due to the thermal movement of molecules in water, some hydrogen bonds are broken and some are formed. Thus, the molecules are mobile in a liquid state, which is very important for metabolic processes. Water molecules easily penetrate cell membranes. Due to the high polarity of its molecules, water is a solvent for other polar compounds. Depending on the ability of certain compounds to dissolve in water, they are conventionally divided into hydrophilic, or polar, and hydrophobic, or non-polar. Most salts are hydrophilic compounds that are soluble in water. Hydrophobic compounds (almost all fats, some proteins) contain non-polar groups that do not form hydrogen bonds, so these compounds are not soluble in water. It has a high heat capacity and at the same time high thermal conductivity for liquids. These properties make water ideal for maintaining thermal balance in the body.
To maintain the vital processes of individual cells and the body as a whole, mineral salts are important. Living organisms contain dissolved salts (in the form of ions) and salts in the solid state. Ions are divided into positive (cations of metal elements K +, N a +, Ca 2+, M 2+, etc.) and negative (hydrochloric acid anions - C l -, sulfate - H SO 4 -, S O 4 2-, carbonate - HCO 3 -, phosphate - H 2 PO 4 -, NPO 4 2-, etc.). Different concentrations of K + and cations N a + in the cell and intercellular fluid causes a potential difference on the cell membrane; change in membrane permeability to K + and N a + under the influence of irritation ensures the occurrence of nervous and muscle excitation. Phosphate acid anions support the neutral reaction of the intracellular environment (pH = 6.9), carboxylic acid anions support the slightly alkaline reaction of the blood plasma (pH = 7.4). Calcium compounds (CaC O 3 ) are part of the shells of mollusks and protozoa, and the shells of crayfish. Chloride acid creates an acidic environment in the stomachvertebrates and humans, thereby ensuring the activity of gastric juice enzymes. Residues of sulfuric acid join water-insoluble compounds, ensuring their solubility, which contributes to the removal of these compounds from cells and the body.
The environment is the totality of living conditions for living beings. The external environment is distinguished, i.e. a complex of factors located outside the body, but necessary for its life, and the internal environment.
The internal environment of the body is the totality of biological fluids (blood, lymph, tissue fluid) that wash cells and tissue structures and take part in metabolic processes. Claude Bernard proposed the concept of “internal environment” in the 19th century, emphasizing that, in contrast to the changing external environment in which a living organism exists, the constancy of the life processes of cells requires a corresponding constancy of their environment, i.e. internal environment.
A living organism is an open system. An open system is a system whose existence requires constant exchange of matter, energy and information with the external environment. The relationship between the body and the external environment ensures the supply of oxygen, water and nutrients to the internal environment, and the removal of carbon dioxide and unnecessary, and sometimes harmful, metabolites. The external environment supplies the body with a huge amount of information perceived by numerous sensitive formations of the nervous system.
The external environment has not only beneficial but also harmful influences on the life of the body. However, a healthy body functions normally if environmental influences do not exceed acceptable limits. This dependence of the life activity of the organism on the external environment, on the one hand, and the relative stability and independence of life processes from changes in the environment, on the other hand, is ensured by the property of the organism, called homeostasis (homeostasis). The body is an ultrastable system that itself searches for the most stable and optimal state, keeping various parameters of functions within the boundaries of physiological (“normal”) fluctuations.
Homeostasis is the relative dynamic constancy of the internal environment and the stability of physiological functions. This is precisely dynamic, and not static, constancy, since it implies not only the possibility, but the necessity of fluctuations in the composition of the internal environment and functional parameters within physiological boundaries in order to achieve the optimal level of vital activity of the organism.
The activity of cells requires an adequate function of supplying them with oxygen and effectively flushing out carbon dioxide and other waste substances or metabolites. To restore decaying protein structures and extract energy, cells must receive plastic and energy material that enters the body with food. Cells receive all this from their surrounding microenvironment through tissue fluid. The constancy of the latter is maintained due to the exchange of gases, ions and molecules with the blood. Consequently, the constancy of blood composition and the state of barriers between blood and tissue fluid, the so-called histohematic barriers, are conditions for homeostasis of the cell microenvironment. The selective permeability of these barriers provides a certain specificity in the composition of the cell microenvironment necessary for their functions.
On the other hand, tissue fluid participates in the formation of lymph and exchanges with lymphatic capillaries draining tissue spaces, which makes it possible to effectively remove large molecules from the cellular microenvironment that are unable to diffuse through histohematic barriers into the blood. In turn, the lymph flowing from the tissues enters the blood through the thoracic lymphatic duct, ensuring the maintenance of a constant composition. Consequently, in the body there is a continuous exchange between the fluids of the internal environment, which is a prerequisite for homeostasis.
The interrelations of the components of the internal environment with each other, with the external environment and the role of the main physiological systems in the implementation of the interaction of the internal and external environment are presented in Fig. 2.1. The external environment influences the body through the perception of its characteristics by the sensitive apparatus of the nervous system (receptors, sensory organs), through the lungs, where gas exchange occurs, and through the gastrointestinal tract, where water and food ingredients are absorbed. The nervous system exerts its regulatory effect on cells due to the release at the ends of nerve conductors of special intermediaries - mediators, which enter through the microenvironment of cells to special structural formations of cell membranes - receptors. The influence of the external environment perceived by the nervous system can also be mediated through the endocrine system, which secretes special humoral regulators - hormones - into the blood. In turn, the substances contained in the blood and tissue fluid, to a greater or lesser extent, irritate the receptors of the interstitial space and the bloodstream, thereby providing the nervous system with information about the composition of the internal environment. Removal of metabolites and foreign substances from the internal environment is carried out through the excretory organs, mainly the kidneys, as well as the lungs and digestive tract.
Constancy of the internal environment is the most important condition for the life of an organism. Therefore, deviations in the composition of liquids in the internal environment are perceived by numerous receptors Fig. 2.1. Scheme of interrelations of the internal environment of the body.
structures and cellular elements with the subsequent inclusion of biochemical, biophysical and physiological regulatory reactions aimed at eliminating the deviation. At the same time, the regulatory reactions themselves cause changes in the internal environment in order to bring it into conformity with the new conditions of existence of the organism. Therefore, regulation of the internal environment always aims to optimize its composition and physiological processes in the body.
The boundaries of homeostatic regulation of the constancy of the internal environment can be rigid for some parameters and flexible for others. Accordingly, the parameters of the internal environment are called rigid constants if the range of their deviations is very small (pH, ion concentration in the blood), or plastic constants (level of glucose, lipids, residual nitrogen, interstitial fluid pressure, etc.), i.e. subject to relatively large fluctuations. Constants vary depending on age, social and professional conditions, time of year and day, geographical and natural conditions, and also have gender and individual characteristics. External environmental conditions are often the same for a larger or smaller number of people living in a certain region and belonging to the same social and age group, but the constants of the internal environment may differ among different healthy people. Thus, homeostatic regulation of the constancy of the internal environment does not mean complete identity of its composition in different individuals. However, despite individual and group characteristics, homeostasis ensures the maintenance of normal parameters of the internal environment of the body.
Typically, the norm refers to the average statistical values of the parameters and characteristics of the vital functions of healthy individuals, as well as the intervals within which fluctuations in these values correspond to homeostasis, i.e. are able to keep the body at the level of optimal functioning.
Accordingly, for a general description of the internal environment of the body, the intervals of fluctuations of its various indicators are usually given, for example, the quantitative content of various substances in the blood of healthy people. At the same time, the characteristics of the internal environment are interrelated and interdependent quantities. Therefore, shifts in one of them are often compensated by others, which does not necessarily affect the level of optimal functioning and human health.
The internal environment is a reflection of the most complex integration of the life activity of different cells, tissues, organs and systems with the influences of the external environment.
This determines the particular importance of the individual characteristics of the internal environment that distinguish each person. The individuality of the internal environment is based on genetic individuality, as well as long-term exposure to certain environmental conditions. Accordingly, the physiological norm is the individual optimum of life activity, i.e. the most coordinated and effective combination of all life processes in real environmental conditions.
2.1. Blood as the internal environment of the body.
Fig.2.2. Main components of blood.
Blood consists of plasma and cells (formed elements) - erythrocytes, leukocytes and platelets, which are in suspension (Fig. 2.2.). Since plasma and cellular elements have separate sources of regeneration, blood is often isolated into an independent type of tissue.
The functions of blood are diverse. This is, first of all, in a generalized form, the function of transport or transfer of gases and substances necessary for the life of cells or to be removed from the body. These include: respiratory, nutritional, integrative-regulatory and excretory functions (see Chapter 6).
Blood also performs a protective function in the body by binding and neutralizing toxic substances that enter the body, binding and destroying foreign protein molecules and foreign cells, including those of infectious origin. Blood is one of the main environments where the body’s specific defense mechanisms against foreign molecules and cells are carried out, i.e. immunity.
Blood is involved in the regulation of all types of metabolism and temperature homeostasis, and is the source of all fluids, secretions and excrements of the body. The composition and properties of blood reflect changes occurring in other internal fluids and cells, and therefore blood tests are the most important diagnostic method.
The amount or volume of blood in a healthy person is within 68% of body weight (4 - 6 liters). This condition is called normovolemia. After excessive intake of water, blood volume may increase (hypervolemia), and during heavy physical work in hot workshops and excessive sweating, it may fall (hypovolemia).
Fig.2.3. Determination of hematocrit.
Since blood consists of cells and plasma, the total volume of blood also consists of the volume of plasma and the volume of cellular elements. The part of the blood volume pertaining to the cellular part of the blood is called hematocrit (Fig. 2.3.). In healthy men, the hematocrit is within 4448%, and in women - 4145%. Due to the presence of numerous mechanisms for regulating blood volume and plasma volume (volumoreceptor reflexes, thirst, nervous and humoral mechanisms for changing the absorption and excretion of water and salts, regulation of the protein composition of the blood, regulation of erythropoiesis, etc.), hematocrit is a relatively rigid homeostatic constant and its long-term and persistent a change is possible only in high altitude conditions, when adaptation to low partial pressure of oxygen enhances erythropoiesis and, accordingly, increases the proportion of blood volume accounted for by cellular elements. Normal values of hematocrit and, accordingly, the volume of cellular elements are called normocythemia. An increase in the volume occupied by blood cells is called polycythemia, and a decrease is called oligocythemia.
Physicochemical properties of blood and plasma. The functions of blood are largely determined by its physicochemical properties, among which the most important are osmotic pressure, oncotic pressure and colloidal stability, suspension stability, specific gravity and viscosity.
The osmotic pressure of the blood depends on the concentration in the blood plasma of the molecules of substances dissolved in it (electrolytes and non-electrolytes) and is the sum of the osmotic pressures of the ingredients contained in it. In this case, over 60% of the osmotic pressure is created by sodium chloride, and in total, inorganic electrolytes account for up to 96% of the total osmotic pressure. Osmotic pressure is one of the rigid homeostatic constants and in a healthy person averages 7.6 atm with a possible range of fluctuations of 7.38.0 atm. If the internal fluid or artificially prepared solution has the same osmotic pressure as normal blood plasma, such a liquid medium or solution is called isotonic. Accordingly, a fluid with a higher osmotic pressure is called hypertonic, and a fluid with a lower one is called hypotonic.
Osmotic pressure ensures the transition of the solvent through a semi-permeable membrane from a less concentrated solution to a more concentrated solution, therefore it plays an important role in the distribution of water between the internal environment and the cells of the body. So, if the tissue fluid is hypertonic, then water will enter it from two sides - from the blood and from the cells; on the contrary, when the extracellular environment is hypotonic, water passes into the cells and blood.
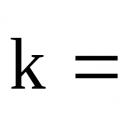 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe