Saturation metal bond. Metal connection
The lesson will cover several types of chemical bonds: metallic, hydrogen and van der Waals, and you will also learn how physical and chemical properties depend on different types chemical bonds in a substance.
Topic: Types of chemical bonds
Lesson: Metal and Hydrogen Chemical Bonds
Metal connection it is a type of bonding in metals and their alloys between metal atoms or ions and relatively free electrons (electron gas) in the crystal lattice.
Metals are chemical elements with low electronegativity, so they easily give up their valence electrons. If there is a non-metal next to a metal element, then electrons from the metal atom go to the non-metal. This type of connection is called ionic(Fig. 1).
Rice. 1. Education
When simple substances metals or their alloys, the situation is changing.
When molecules are formed, the electron orbitals of metals do not remain unchanged. They interact with each other, forming a new molecular orbital. Depending on the composition and structure of the compound, molecular orbitals can be either close to the totality of atomic orbitals or significantly different from them. When the electron orbitals of metal atoms interact, molecular orbitals are formed. Such that the valence electrons of the metal atom can move freely through these molecular orbitals. Complete separation of charge does not occur, i.e. metal- this is not a collection of cations and electrons floating around. But this is not a collection of atoms that sometimes transform into a cationic form and transfer their electron to another cation. The real situation is a combination of these two extreme options.

Rice. 2
The essence of metal bond formation consists of as follows: metal atoms donate outer electrons, and some of them turn into positively charged ions. Torn away from atoms electrons move relatively freely between emerging positivemetal ions. A metallic bond arises between these particles, i.e., electrons seem to cement positive ions in the metal lattice (Fig. 2).
The presence of a metallic bond determines the physical properties of metals:
High ductility
Heat and electrical conductivity
Metallic shine
Plastic - this is the ability of a material to easily deform under mechanical load. A metallic bond is realized between all metal atoms simultaneously, therefore, when a metal is subjected to mechanical action, specific bonds are not broken, but only the position of the atom changes. Metal atoms, not connected by rigid bonds to each other, can, as it were, slide along a layer of electron gas, as happens when one glass slides over another with a layer of water between them. Thanks to this, metals can be easily deformed or rolled into thin foil. The most ductile metals are pure gold, silver and copper. All these metals are found in nature in native form in varying degrees of purity. Rice. 3.

Rice. 3. Metals found in nature in native form
Various jewelry is made from them, especially gold. Due to its amazing plasticity, gold is used in the decoration of palaces. You can roll out foil from it to a thickness of only 3. 10 -3 mm. It is called gold leaf and is applied to plaster, moldings or other objects.
Thermal and electrical conductivity . The best thing electricity conduct copper, silver, gold and aluminum. But since gold and silver are expensive metals, cheaper copper and aluminum are used to make cables. The worst electrical conductors are manganese, lead, mercury and tungsten. Tungsten has such high electrical resistance that when an electric current passes through it, it begins to glow. This property is used in the manufacture of incandescent lamps.
Body temperature is a measure of the energy of its constituent atoms or molecules. The electron gas of a metal can transfer excess energy quite quickly from one ion or atom to another. The temperature of the metal quickly equalizes throughout the entire volume, even if heating occurs on one side. This is observed, for example, if you dip a metal spoon into tea.
Metallic shine. Gloss is the ability of a body to reflect light rays. Silver, aluminum and palladium have high light reflectivity. Therefore, it is these metals that are applied in a thin layer to the glass surface in the manufacture of headlights, spotlights and mirrors.
Hydrogen bond
Consider the boiling and melting points hydrogen compounds chalcogens: oxygen, sulfur, selenium and tellurium. Rice. 4.

Rice. 4
If we mentally extrapolate the direct boiling and melting temperatures of hydrogen compounds of sulfur, selenium and tellurium, we will see that the melting point of water should be approximately -100 0 C, and the boiling point - approximately -80 0 C. This happens because there is a gap between water molecules interaction - hydrogen bond, which unites water molecules to the association . Additional energy is required to destroy these associates.
A hydrogen bond is formed between a highly polarized, highly positively charged hydrogen atom and another atom with very high electronegativity: fluorine, oxygen or nitrogen . Examples of substances capable of forming hydrogen bonds are shown in Fig. 5.

Rice. 5
Consider the formation of hydrogen bonds between water molecules. A hydrogen bond is represented by three dots. The occurrence of a hydrogen bond is due to the unique feature of the hydrogen atom. Since the hydrogen atom contains only one electron, when a common electron pair is pulled away by another atom, the nucleus of the hydrogen atom is exposed, the positive charge of which acts on the electronegative elements in the molecules of substances.

Let's compare the properties ethyl alcohol and dimethyl ether. Based on the structure of these substances, it follows that ethyl alcohol can form intermolecular hydrogen bonds. This is due to the presence of a hydroxo group. Dimethyl ether cannot form intermolecular hydrogen bonds.
Let's compare their properties in Table 1.
Table 1
Boiling point, mp., solubility in water is higher for ethyl alcohol. This general pattern for substances whose molecules form a hydrogen bond. These substances are characterized by higher boiling point, melting temperature, solubility in water and lower volatility.
Physical properties compounds also depend on the molecular weight of the substance. Therefore, it is legitimate to compare the physical properties of substances with hydrogen bonds only for substances with similar molecular masses.
Energy one hydrogen bond about 10 times less energy covalent bond . If organic molecules of complex composition have several functional groups capable of forming hydrogen bonds, then intramolecular hydrogen bonds can form in them (proteins, DNA, amino acids, orthonitrophenol, etc.). Due to hydrogen bonding, it is formed secondary structure proteins, DNA double helix.
Van der Waals connection.
Let's remember the noble gases. Helium compounds have not yet been obtained. It is not capable of forming ordinary chemical bonds.
At very low temperatures, liquid and even solid helium can be obtained. In the liquid state, helium atoms are held together by the forces of electrostatic attraction. There are three variants of these powers:
· orientation forces. This is the interaction between two dipoles (HCl)
· inductive attraction. This is the attraction between a dipole and a nonpolar molecule.
· dispersion attraction. This is the interaction between two non-polar molecules (He). It occurs due to the uneven movement of electrons around the nucleus.
Summing up the lesson
The lesson covers three types of chemical bonds: metallic, hydrogen and van der Waals. The dependence of physical and chemical properties from different types of chemical bonds in a substance.
Bibliography
1. Rudzitis G.E. Chemistry. Basics general chemistry. 11th grade: textbook for educational institutions: a basic level of/ G.E. Rudzitis, F.G. Feldman. - 14th ed. - M.: Education, 2012.
2. Popel P.P. Chemistry: 8th grade: textbook for general education educational institutions/ P.P. Popel, L.S. Krivlya. - K.: IC "Academy", 2008. - 240 pp.: ill.
3. Gabrielyan O.S. Chemistry. Grade 11. A basic level of. 2nd ed., erased. - M.: Bustard, 2007. - 220 p.
Homework
1. No. 2, 4, 6 (p. 41) Rudzitis G.E. Chemistry. Fundamentals of general chemistry. 11th grade: textbook for general education institutions: basic level / G.E. Rudzitis, F.G. Feldman. - 14th ed. - M.: Education, 2012.
2. Why is tungsten used to make filaments of incandescent lamps?
3. What explains the absence of hydrogen bonds in aldehyde molecules?
Metal connection. Properties of metallic bond.
A metallic bond is a chemical bond caused by the presence of relatively free electrons. Characteristic of both pure metals and their alloys and intermetallic compounds.
Metal link mechanism
Positive metal ions are located at all nodes of the crystal lattice. Between them, valence electrons move randomly, like gas molecules, detached from the atoms during the formation of ions. These electrons act as cement, holding the positive ions together; otherwise, the lattice would disintegrate under the influence of repulsive forces between the ions. At the same time, electrons are held by ions within the crystal lattice and cannot leave it. The coupling forces are not localized or directed. For this reason, in most cases high coordination numbers appear (for example, 12 or 8). When two metal atoms come close together, the orbitals in their outer shells overlap to form molecular orbitals. If a third atom approaches, its orbital overlaps with the orbitals of the first two atoms, resulting in another molecular orbital. When there are many atoms, a huge number of three-dimensional molecular orbitals arise, extending in all directions. Due to multiple overlapping orbitals, the valence electrons of each atom are influenced by many atoms.
Characteristic crystal lattices
Most metals form one of the following highly symmetrical lattices with close packing of atoms: body-centered cubic, face-centered cubic, and hexagonal.
In a body-centered cubic (bcc) lattice, the atoms are located at the vertices of the cube and one atom is at the center of the cube volume. Metals have a cubic body-centered lattice: Pb, K, Na, Li, β-Ti, β-Zr, Ta, W, V, α-Fe, Cr, Nb, Ba, etc.
In a face-centered cubic (fcc) lattice, the atoms are located at the vertices of the cube and at the center of each face. Metals of this type have a lattice: α-Ca, Ce, α-Sr, Pb, Ni, Ag, Au, Pd, Pt, Rh, γ-Fe, Cu, α-Co, etc.
In a hexagonal lattice, the atoms are located at the vertices and center of the hexagonal bases of the prism, and three atoms are located in the middle plane of the prism. Metals have this packing of atoms: Mg, α-Ti, Cd, Re, Os, Ru, Zn, β-Co, Be, β-Ca, etc.
Other properties
Freely moving electrons cause high electrical and thermal conductivity. Substances that have a metallic bond often combine strength with plasticity, since when atoms are displaced relative to each other, the bonds do not break. Also important property is metallic aromaticity.
Metals conduct heat and electricity well, they are strong enough, and can be deformed without destruction. Some metals are malleable (they can be forged), some are malleable (you can draw wire from them). These unique properties are explained by a special type of chemical bond that connects metal atoms to each other - a metallic bond.
Metals in the solid state exist in the form of crystals of positive ions, as if “floating” in a sea of electrons freely moving between them.
Metallic bond explains the properties of metals, in particular their strength. Under the influence of a deforming force, a metal lattice can change its shape without cracking, unlike ionic crystals.
The high thermal conductivity of metals is explained by the fact that if a piece of metal is heated on one side, the kinetic energy of the electrons will increase. This increase in energy will spread in the “electron sea” throughout the sample at high speed.
The electrical conductivity of metals also becomes clear. If a potential difference is applied to the ends of a metal sample, the cloud of delocalized electrons will shift in the direction of the positive potential: this flow of electrons moving in one direction represents the familiar electric current.
Metal connection. Properties of metallic bond. - concept and types. Classification and features of the category "Metallic bond. Properties of metallic bond." 2017, 2018.
Metal connection
As a result of electrostatic attraction between the cation and anion, a molecule is formed.
The theory of ionic bonding was proposed by 1916 ᴦ. German scientist W. Kossel. This theory explains the formation of connections between atoms of typical metals and atoms typical non-metals: CsF, CsCl, NaCl, KF, KCl, Na 2 O, etc.
According to this theory, when an ionic bond is formed, atoms of typical metals give up electrons, and atoms of typical nonmetals accept electrons.
As a result of these processes, metal atoms are transformed into positively charged particles, which are called positive ions or cations; and non-metal atoms turn into negative ions - anions. The charge of the cation is equal to the number of electrons given up.
Metal atoms donate electrons to their outer layer, and the resulting ions have complete electronic structures (pre-outer electronic layer).
The magnitude of the negative charge of the anion is equal to the number of electrons accepted.
Non-metal atoms accept the number of electrons that is extremely important for them to completion of an electronic octet (outer electronic layer).
For example: the general scheme for the formation of a NaCl molecule from Na and C1 atoms: Na°-le = Na +1 Formation of ions
Сl°+1е - = Сl -
Na +1 + Cl - = Na + Cl -
Na°+ Сl°= Na + Сl - Compound of ions
· The bond between ions is commonly called ionic bonding.
Compounds that consist of ions are called ionic compounds.
The algebraic sum of the charges of all ions in the molecule of an ionic compound must be equal to zero, because any molecule is an electrically neutral particle.
There is no sharp boundary between ionic and covalent bonds. An ionic bond can be considered as an extreme case of a polar covalent bond, in which the formation of a shared electron pair completely moves towards the atom with higher electronegativity.
Most typical metal atoms have a small number of electrons in their outer electron layer (typically 1 to 3); these electrons are called valence electrons. In metal atoms, the strength of the bond between the valence electrons and the nucleus is low, that is, the atoms have low ionization energy. This makes it easy to lose valence electrons h transformation of metal atoms into positively charged ions (cations):
Ме° -ne ® Ме n +
In the crystal structure of a metal, valence electrons have the ability to easily move from one atom to another, which leads to the sharing of electrons by all neighboring atoms. In a simplified way, the structure of a metal crystal is represented as follows: at the nodes of the crystal lattice there are Me n+ ions and Me° atoms, and valence electrons move relatively freely between them, establishing connections between all atoms and ions of the metal (Fig. 3). This is a special type of chemical bond called a metal bond.
· Metallic bond - a bond between atoms and ions of metals in a crystal lattice, carried out by shared valence electrons.
Thanks to this type of chemical bond, metals have a certain set of physical and chemical properties that distinguish them from non-metals.
 Rice. 3. Diagram of the crystal lattice of metals.
Rice. 3. Diagram of the crystal lattice of metals.
The strength of the metal bond ensures the stability of the crystal lattice and the plasticity of metals (the ability to undergo various processing without destruction). The free movement of valence electrons allows metals to conduct electricity and heat well. The ability to reflect light waves (ᴛ.ᴇ. metallic luster) is also explained by the structure of the metal’s crystal lattice.
However, the most characteristic physical properties of metals based on the presence of a metallic bond are:
■metallic luster and opacity;
■plasticity, malleability, fusibility;
■high electrical and thermal conductivity; and a tendency to form alloys.
Metal bond - concept and types. Classification and features of the category "Metal connection" 2017, 2018.
The very name “metallic bond” indicates that we are talking about the internal structure of metals. The atoms of most metals at the outer energy level contain a small number of valence electrons compared to total number external energetically close... .
The metallic bond is based on the sharing of valence electrons belonging not to two, but to almost all metal atoms in the crystal. In metals, there are much fewer valence electrons than free orbitals. This creates conditions for free movement... .
Essential information regarding the nature of chemical bonds in metals can be obtained on the basis of two characteristic features compared to covalent and ionic compounds. Metals, firstly, differ from other substances in their high electrical conductivity and... .
Significant information about the nature of chemical bonds in metals can be obtained on the basis of two characteristic features of them in comparison with covalent and ionic compounds. Metals, firstly, differ from other substances in their high electrical conductivity and... .
Hybridization of orbitals and spatial configuration of molecules Type of molecule Initial orbitals of atom A Type of hybridization Number of hybrid orbitals of atom A Spatial configuration of the molecule AB2 AB3 AB4 s + p s + p + p s + p + p + p sp sp2 sp3 ... .
A metallic bond is a chemical bond caused by the presence of relatively free electrons. Characteristic of both pure metals and their alloys and intermetallic compounds. Mechanism of metallic bonding At all nodes of the crystal lattice there are... .
A molecule is the smallest particle of a substance that has its chemical properties. According to the theory of chemical bonding, the stable state of an element corresponds to a structure with the electronic formula of the outer level s2p6 (argon, krypton, radon, and others). During education... .
Topics of the Unified State Examination codifier: Covalent chemical bond, its varieties and mechanisms of formation. Characteristics of covalent bonds (polarity and bond energy). Ionic bond. Metal connection. Hydrogen bond
Intramolecular chemical bonds
First, let's look at the bonds that arise between particles within molecules. Such connections are called intramolecular.
Chemical bond between atoms chemical elements has an electrostatic nature and is formed due to interaction of external (valence) electrons, in more or less degree held by positively charged nuclei bonded atoms.
The key concept here is ELECTRONEGATIVITY. It is this that determines the type of chemical bond between atoms and the properties of this bond.
is the ability of an atom to attract (hold) external(valence) electrons. Electronegativity is determined by the degree of attraction of outer electrons to the nucleus and depends primarily on the radius of the atom and the charge of the nucleus.
Electronegativity is difficult to determine unambiguously. L. Pauling compiled a table of relative electronegativities (based on the bond energies of diatomic molecules). The most electronegative element is fluorine with meaning 4 .
It is important to note that in different sources you can find different scales and tables of electronegativity values. This should not be alarmed, since the formation of a chemical bond plays a role atoms, and it is approximately the same in any system.
If one of the atoms in the A:B chemical bond attracts electrons more strongly, then the electron pair moves towards it. The more electronegativity difference atoms, the more the electron pair shifts.
If the electronegativities of interacting atoms are equal or approximately equal: EO(A)≈EO(B), then the common electron pair does not shift to any of the atoms: A: B. This connection is called covalent nonpolar.
If the electronegativities of the interacting atoms differ, but not greatly (the difference in electronegativity is approximately from 0.4 to 2: 0,4<ΔЭО<2 ), then the electron pair is displaced to one of the atoms. This connection is called covalent polar .
If the electronegativities of interacting atoms differ significantly (the difference in electronegativity is greater than 2: ΔEO>2), then one of the electrons is almost completely transferred to another atom, with the formation ions. This connection is called ionic.
Basic types of chemical bonds − covalent, ionic And metal communications. Let's take a closer look at them.
Covalent chemical bond
Covalent bond – it's a chemical bond , formed due to formation of a common electron pair A:B . Moreover, two atoms overlap atomic orbitals. A covalent bond is formed by the interaction of atoms with a small difference in electronegativity (usually between two non-metals) or atoms of one element.
Basic properties of covalent bonds
- focus,
- saturability,
- polarity,
- polarizability.
These bonding properties influence the chemical and physical properties of substances.
Communication direction characterizes the chemical structure and form of substances. The angles between two bonds are called bond angles. For example, in a water molecule the bond angle H-O-H is 104.45 o, therefore the water molecule is polar, and in a methane molecule the bond angle H-C-H is 108 o 28′.

Saturability is the ability of atoms to form a limited number of covalent chemical bonds. The number of bonds that an atom can form is called.
Polarity bonding occurs due to the uneven distribution of electron density between two atoms with different electronegativity. Covalent bonds are divided into polar and nonpolar.
Polarizability connections are the ability of bond electrons to shift under the influence of an external electric field(in particular, the electric field of another particle). Polarizability depends on electron mobility. The further the electron is from the nucleus, the more mobile it is, and accordingly the molecule is more polarizable.

Covalent nonpolar chemical bond
There are 2 types of covalent bonding – POLAR And NON-POLAR .
Example . Let's consider the structure of the hydrogen molecule H2. Each hydrogen atom in its outer energy level carries 1 unpaired electron. To display an atom, we use the Lewis structure - this is a diagram of the structure of the outer energy level of an atom, when electrons are indicated by dots. Lewis point structure models are quite helpful when working with elements of the second period.
H. + . H = H:H
Thus, a hydrogen molecule has one shared electron pair and one H–H chemical bond. This electron pair does not shift to any of the hydrogen atoms, because Hydrogen atoms have the same electronegativity. This connection is called covalent nonpolar .

Covalent nonpolar (symmetric) bond is a covalent bond formed by atoms with equal electronegativity (usually the same nonmetals) and, therefore, with a uniform distribution of electron density between the nuclei of atoms.
The dipole moment of non-polar bonds is 0.
Examples: H 2 (H-H), O 2 (O=O), S 8.
Covalent polar chemical bond
Covalent polar bond is a covalent bond that occurs between atoms with different electronegativity (usually, various non-metals) and is characterized displacement shared electron pair to a more electronegative atom (polarization).
The electron density is shifted to the more electronegative atom - therefore, a partial negative charge (δ-) appears on it, and a partial positive charge (δ+, delta +) appears on the less electronegative atom.
The greater the difference in electronegativity of atoms, the higher polarity connections and more dipole moment . Additional attractive forces act between neighboring molecules and charges of opposite sign, which increases strength communications.
Bond polarity affects the physical and chemical properties of compounds. The reaction mechanisms and even the reactivity of neighboring bonds depend on the polarity of the bond. The polarity of the connection often determines molecule polarity and thus directly affects such physical properties as boiling point and melting point, solubility in polar solvents.
Examples: HCl, CO 2, NH 3.
Mechanisms of covalent bond formation
Covalent chemical bonds can occur by 2 mechanisms:
1. Exchange mechanism the formation of a covalent chemical bond is when each particle provides one unpaired electron to form a common electron pair:
A . + . B= A:B
2. Covalent bond formation is a mechanism in which one of the particles provides a lone pair of electrons, and the other particle provides a vacant orbital for this electron pair:
A: + B= A:B

In this case, one of the atoms provides a lone pair of electrons ( donor), and the other atom provides a vacant orbital for that pair ( acceptor). As a result of the formation of both bonds, the energy of the electrons decreases, i.e. this is beneficial for the atoms.
A covalent bond formed by a donor-acceptor mechanism is not different in properties from other covalent bonds formed by the exchange mechanism. The formation of a covalent bond by the donor-acceptor mechanism is typical for atoms either with a large number of electrons at the external energy level (electron donors), or, conversely, with a very small number of electrons (electron acceptors). The valence capabilities of atoms are discussed in more detail in the corresponding section.
A covalent bond is formed by a donor-acceptor mechanism:
- in a molecule carbon monoxide CO(the bond in the molecule is triple, 2 bonds are formed by the exchange mechanism, one by the donor-acceptor mechanism): C≡O;
- V ammonium ion NH 4 +, in ions organic amines, for example, in the methylammonium ion CH 3 -NH 2 + ;
- V complex compounds, a chemical bond between the central atom and ligand groups, for example, in sodium tetrahydroxoaluminate Na bond between aluminum and hydroxide ions;
- V nitric acid and its salts- nitrates: HNO 3, NaNO 3, in some other nitrogen compounds;

- in a molecule ozone O3.
Basic characteristics of covalent bonds
Covalent bonds typically form between nonmetal atoms. The main characteristics of a covalent bond are length, energy, multiplicity and directionality.
Multiplicity of chemical bond
Multiplicity of chemical bond - This number of shared electron pairs between two atoms in a compound. The multiplicity of a bond can be determined quite easily from the values of the atoms that form the molecule.
For example , in the hydrogen molecule H 2 the bond multiplicity is 1, because Each hydrogen has only 1 unpaired electron in its outer energy level, hence one shared electron pair is formed.
In the O 2 oxygen molecule, the bond multiplicity is 2, because Each atom at the outer energy level has 2 unpaired electrons: O=O.
In the nitrogen molecule N2, the bond multiplicity is 3, because between each atom there are 3 unpaired electrons at the outer energy level, and the atoms form 3 common electron pairs N≡N.
Covalent bond length
Chemical bond length
is the distance between the centers of the nuclei of the atoms forming the bond. It is determined by experimental physical methods. The bond length can be estimated approximately using the additivity rule, according to which the bond length in the AB molecule is approximately equal to half the sum of the bond lengths in molecules A 2 and B 2: 
The length of a chemical bond can be roughly estimated by atomic radii forming a bond, or by communication multiplicity, if the radii of the atoms are not very different.
As the radii of the atoms forming a bond increase, the bond length will increase.
For example
As the multiplicity of bonds between atoms increases (the atomic radii of which do not differ or differ only slightly), the bond length will decrease.
For example . In the series: C–C, C=C, C≡C, the bond length decreases.
Communication energy
A measure of the strength of a chemical bond is the bond energy. Communication energy determined by the energy required to break a bond and remove the atoms forming that bond to an infinitely large distance from each other.
A covalent bond is very durable. Its energy ranges from several tens to several hundred kJ/mol. The higher the bond energy, the greater the bond strength, and vice versa.
The strength of a chemical bond depends on the bond length, bond polarity, and bond multiplicity. The longer a chemical bond, the easier it is to break, and the lower the bond energy, the lower its strength. The shorter the chemical bond, the stronger it is, and the greater the bond energy.
For example, in the series of compounds HF, HCl, HBr from left to right, the strength of the chemical bond decreases, because The connection length increases.
Ionic chemical bond

Ionic bond is a chemical bond based on electrostatic attraction of ions.
Ions are formed in the process of accepting or donating electrons by atoms. For example, atoms of all metals weakly hold electrons from the outer energy level. Therefore, metal atoms are characterized by restorative properties- ability to donate electrons.

Example. The sodium atom contains 1 electron at energy level 3. By easily giving it up, the sodium atom forms the much more stable Na + ion, with the electron configuration of the noble gas neon Ne. The sodium ion contains 11 protons and only 10 electrons, so the total charge of the ion is -10+11 = +1:
+11Na) 2 ) 8 ) 1 - 1e = +11 Na +) 2 ) 8
Example. A chlorine atom in its outer energy level contains 7 electrons. To acquire the configuration of a stable inert argon atom Ar, chlorine needs to gain 1 electron. After adding an electron, a stable chlorine ion is formed, consisting of electrons. The total charge of the ion is -1:
+17Cl) 2 ) 8 ) 7 + 1e = +17 Cl — ) 2 ) 8 ) 8
Note:
- The properties of ions are different from the properties of atoms!
- Stable ions can form not only atoms, but also groups of atoms. For example: ammonium ion NH 4 +, sulfate ion SO 4 2-, etc. Chemical bonds formed by such ions are also considered ionic;
- Ionic bonds are usually formed between each other metals And nonmetals(non-metal groups);
The resulting ions are attracted due to electrical attraction: Na + Cl -, Na 2 + SO 4 2-.
Let us visually summarize difference between covalent and ionic bond types:


Metal connection is a connection that is formed relatively free electrons between metal ions, forming a crystal lattice.
Metal atoms are usually located on the outer energy level one to three electrons. The radii of metal atoms, as a rule, are large - therefore, metal atoms, unlike non-metals, give up their outer electrons quite easily, i.e. are strong reducing agents.
By donating electrons, metal atoms turn into positively charged ions . The detached electrons are relatively free are moving between positively charged metal ions. Between these particles a connection arises, because shared electrons hold metal cations arranged in layers together , thus creating a fairly strong metal crystal lattice . In this case, the electrons continuously move chaotically, i.e. New neutral atoms and new cations constantly appear.

Intermolecular interactions
Separately, it is worth considering the interactions that arise between individual molecules in a substance - intermolecular interactions . Intermolecular interactions are a type of interaction between neutral atoms in which no new covalent bonds appear. The forces of interaction between molecules were discovered by Van der Waals in 1869, and named after him Van dar Waals forces. Van der Waals forces are divided into orientation, induction And dispersive . The energy of intermolecular interactions is much less than the energy of chemical bonds.
Orientation forces of attraction occur between polar molecules (dipole-dipole interaction). These forces occur between polar molecules. Inductive interactions is the interaction between a polar molecule and a non-polar one. A nonpolar molecule is polarized due to the action of a polar one, which generates additional electrostatic attraction.
A special type of intermolecular interaction is hydrogen bonds. - these are intermolecular (or intramolecular) chemical bonds that arise between molecules that have highly polar covalent bonds - H-F, H-O or H-N. If there are such bonds in a molecule, then between the molecules there will be additional attractive forces .
Education mechanism hydrogen bonding is partly electrostatic and partly donor-acceptor. In this case, the electron pair donor is an atom of a strongly electronegative element (F, O, N), and the acceptor is the hydrogen atoms connected to these atoms. Hydrogen bonds are characterized by focus in space and saturation
Hydrogen bonds can be indicated by dots: H ··· O. The greater the electronegativity of the atom connected to hydrogen, and the smaller its size, the stronger the hydrogen bond. It is typical primarily for connections fluorine with hydrogen , as well as to oxygen and hydrogen , less nitrogen with hydrogen .

Hydrogen bonds occur between the following substances:
— hydrogen fluoride HF(gas, solution of hydrogen fluoride in water - hydrofluoric acid), water H 2 O (steam, ice, liquid water):
— solution of ammonia and organic amines- between ammonia and water molecules;
— organic compounds in which O-H or N-H bonds: alcohols, carboxylic acids, amines, amino acids, phenols, aniline and its derivatives, proteins, solutions of carbohydrates - monosaccharides and disaccharides.
Hydrogen bonding affects the physical and chemical properties of substances. Thus, additional attraction between molecules makes it difficult for substances to boil. Substances with hydrogen bonds exhibit an abnormal increase in boiling point.
For example As a rule, with increasing molecular weight, an increase in the boiling point of substances is observed. However, in a number of substances H 2 O-H 2 S-H 2 Se-H 2 Te we do not observe a linear change in boiling points.

Namely, at water boiling point is abnormally high - no less than -61 o C, as the straight line shows us, but much more, +100 o C. This anomaly is explained by the presence of hydrogen bonds between water molecules. Therefore, under normal conditions (0-20 o C) water is liquid by phase state.
Chemical bond
All interactions leading to the combination of chemical particles (atoms, molecules, ions, etc.) into substances are divided into chemical bonds and intermolecular bonds (intermolecular interactions).
Chemical bonds- bonds directly between atoms. There are ionic, covalent and metallic bonds.
Intermolecular bonds- connections between molecules. These are hydrogen bonds, ion-dipole bonds (due to the formation of this bond, for example, the formation of a hydration shell of ions occurs), dipole-dipole (due to the formation of this bond, molecules of polar substances are combined, for example, in liquid acetone), etc.
Ionic bond- a chemical bond formed due to the electrostatic attraction of oppositely charged ions. In binary compounds (compounds of two elements), it is formed when the sizes of the bonded atoms are very different from each other: some atoms are large, others are small - that is, some atoms easily give up electrons, while others tend to accept them (usually these are atoms of the elements that form typical metals and atoms of elements forming typical nonmetals); the electronegativity of such atoms is also very different.
Ionic bonding is non-directional and non-saturable.
Covalent bond- a chemical bond that occurs due to the formation of a common pair of electrons. A covalent bond is formed between small atoms with the same or similar radii. A necessary condition is the presence of unpaired electrons in both bonded atoms (exchange mechanism) or a lone pair in one atom and a free orbital in the other (donor-acceptor mechanism):
| A) | H· + ·H H:H | H-H | H 2 | (one shared pair of electrons; H is monovalent); |
| b) | NN | N 2 | (three shared pairs of electrons; N is trivalent); | |
| V) | H-F | HF | (one shared pair of electrons; H and F are monovalent); | |
| G) | NH4+ | (four shared pairs of electrons; N is tetravalent) |
- Based on the number of shared electron pairs, covalent bonds are divided into
- simple (single)- one pair of electrons,
- double- two pairs of electrons,
- triples- three pairs of electrons.
Double and triple bonds are called multiple bonds.
According to the distribution of electron density between the bonded atoms, a covalent bond is divided into non-polar And polar. A non-polar bond is formed between identical atoms, a polar one - between different ones.
Electronegativity- a measure of the ability of an atom in a substance to attract common electron pairs.
The electron pairs of polar bonds are shifted towards more electronegative elements. The displacement of electron pairs itself is called bond polarization. The partial (excess) charges formed during polarization are designated + and -, for example: .
Based on the nature of the overlap of electron clouds ("orbitals"), a covalent bond is divided into -bond and -bond.
-A bond is formed due to the direct overlap of electron clouds (along the straight line connecting the atomic nuclei), -a bond is formed due to lateral overlap (on both sides of the plane in which the atomic nuclei lie).
A covalent bond is directional and saturable, as well as polarizable.
The hybridization model is used to explain and predict the mutual direction of covalent bonds.
Hybridization of atomic orbitals and electron clouds- the supposed alignment of atomic orbitals in energy, and electron clouds in shape when an atom forms covalent bonds.
The three most common types of hybridization are: sp-, sp 2 and sp 3 -hybridization. For example:
sp-hybridization - in molecules C 2 H 2, BeH 2, CO 2 (linear structure);
sp 2-hybridization - in molecules C 2 H 4, C 6 H 6, BF 3 (flat triangular shape);
sp 3-hybridization - in molecules CCl 4, SiH 4, CH 4 (tetrahedral form); NH 3 (pyramidal shape); H 2 O (angular shape).
Metal connection- a chemical bond formed by sharing the valence electrons of all bonded atoms of a metal crystal. As a result, a single electron cloud of the crystal is formed, which easily moves under the influence of electrical voltage - hence the high electrical conductivity of metals.
A metallic bond is formed when the atoms being bonded are large and therefore tend to give up electrons. Simple substances with a metallic bond are metals (Na, Ba, Al, Cu, Au, etc.), complex substances are intermetallic compounds (AlCr 2, Ca 2 Cu, Cu 5 Zn 8, etc.).
The metal bond does not have directionality or saturation. It is also preserved in metal melts.
Hydrogen bond- an intermolecular bond formed due to the partial acceptance of a pair of electrons from a highly electronegative atom by a hydrogen atom with a large positive partial charge. It is formed in cases where one molecule contains an atom with a lone pair of electrons and high electronegativity (F, O, N), and the other contains a hydrogen atom bound by a highly polar bond to one of such atoms. Examples of intermolecular hydrogen bonds:
H—O—H OH 2 , H—O—H NH 3 , H—O—H F—H, H—F H—F.
Intramolecular hydrogen bonds exist in the molecules of polypeptides, nucleic acids, proteins, etc.
A measure of the strength of any bond is the bond energy.
Communication energy- the energy required to break a given chemical bond in 1 mole of a substance. The unit of measurement is 1 kJ/mol.
The energies of ionic and covalent bonds are of the same order, the energy of hydrogen bonds is an order of magnitude lower.
The energy of a covalent bond depends on the size of the bonded atoms (bond length) and on the multiplicity of the bond. The smaller the atoms and the greater the bond multiplicity, the greater its energy.
The ionic bond energy depends on the size of the ions and their charges. The smaller the ions and the greater their charge, the greater the binding energy.
Structure of matter
According to the type of structure, all substances are divided into molecular And non-molecular. Among organic substances, molecular substances predominate, among inorganic substances, non-molecular substances predominate.
Based on the type of chemical bond, substances are divided into substances with covalent bonds, substances with ionic bonds (ionic substances) and substances with metallic bonds (metals).
Substances with covalent bonds can be molecular or non-molecular. This significantly affects their physical properties.
Molecular substances consist of molecules connected to each other by weak intermolecular bonds, these include: H 2, O 2, N 2, Cl 2, Br 2, S 8, P 4 and other simple substances; CO 2, SO 2, N 2 O 5, H 2 O, HCl, HF, NH 3, CH 4, C 2 H 5 OH, organic polymers and many other substances. These substances do not have high strength, have low melting and boiling points, do not conduct electricity, and some of them are soluble in water or other solvents.
Non-molecular substances with covalent bonds or atomic substances (diamond, graphite, Si, SiO 2, SiC and others) form very strong crystals (with the exception of layered graphite), they are insoluble in water and other solvents, have high melting and boiling points, most of them they do not conduct electric current (except for graphite, which is electrically conductive, and semiconductors - silicon, germanium, etc.)
All ionic substances are naturally non-molecular. These are solid, refractory substances, solutions and melts of which conduct electric current. Many of them are soluble in water. It should be noted that in ionic substances, the crystals of which consist of complex ions, there are also covalent bonds, for example: (Na +) 2 (SO 4 2-), (K +) 3 (PO 4 3-), (NH 4 + )(NO 3-), etc. The atoms that make up complex ions are connected by covalent bonds.
Metals (substances with metallic bonds) very diverse in their physical properties. Among them there are liquid (Hg), very soft (Na, K) and very hard metals (W, Nb).
The characteristic physical properties of metals are their high electrical conductivity (unlike semiconductors, it decreases with increasing temperature), high heat capacity and ductility (for pure metals).
In the solid state, almost all substances are composed of crystals. Based on the type of structure and type of chemical bond, crystals (“crystal lattices”) are divided into atomic(crystals of non-molecular substances with covalent bonds), ionic(crystals of ionic substances), molecular(crystals of molecular substances with covalent bonds) and metal(crystals of substances with a metallic bond).
Tasks and tests on the topic "Topic 10. "Chemical bonding. Structure of matter."
- Types of chemical bond - Structure of matter grade 8–9
Lessons: 2 Assignments: 9 Tests: 1
- Assignments: 9 Tests: 1
After working through this topic, you should understand the following concepts: chemical bond, intermolecular bond, ionic bond, covalent bond, metallic bond, hydrogen bond, simple bond, double bond, triple bond, multiple bonds, non-polar bond, polar bond, electronegativity, bond polarization , - and -bond, hybridization of atomic orbitals, binding energy.
You must know the classification of substances by type of structure, by type of chemical bond, the dependence of the properties of simple and complex substances on the type of chemical bond and the type of “crystal lattice”.
You must be able to: determine the type of chemical bond in a substance, the type of hybridization, draw up diagrams of bond formation, use the concept of electronegativity, a number of electronegativity; know how electronegativity changes in chemical elements of the same period and one group to determine the polarity of a covalent bond.
After making sure that everything you need has been learned, proceed to completing the tasks. We wish you success.
Recommended reading:
- O. S. Gabrielyan, G. G. Lysova. Chemistry 11th grade. M., Bustard, 2002.
- G. E. Rudzitis, F. G. Feldman. Chemistry 11th grade. M., Education, 2001.
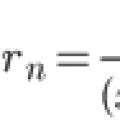 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A