Study of the physical properties of benzene. Aromatic hydrocarbons
Aromatic HCs (arenas)– these are hydrocarbons whose molecules contain one or more benzene rings.
Examples of aromatic hydrocarbons:

Arenas of the benzene series (monocyclic arenes)
General formula:C n H 2n-6 , n≥6
The simplest representative of aromatic hydrocarbons is benzene, its empirical formula is C 6 H 6.
Electronic structure of the benzene molecule
The general formula of monocyclic arenes C n H 2 n -6 shows that they are unsaturated compounds.
In 1856, the German chemist A.F. Kekule proposed a cyclic formula for benzene with conjugated bonds (single and double bonds alternate) - cyclohexatriene-1,3,5: 
This structure of the benzene molecule did not explain many of the properties of benzene:
- Benzene is characterized by substitution reactions rather than addition reactions characteristic of unsaturated compounds. Addition reactions are possible, but are more difficult than for ;
- benzene does not enter into reactions that are qualitative reactions to unsaturated hydrocarbons (with bromine water and KMnO 4 solution).
Later electron diffraction studies showed that all bonds between the carbon atoms in the benzene molecule have the same length of 0.140 nm (the average value between the length of a simple S-S connections 0.154 nm and double bond C=C 0.134 nm). The angle between the bonds at each carbon atom is 120 o. The molecule is a regular flat hexagon.
Modern theory to explain the structure of the C 6 H 6 molecule uses the idea of hybridization of atomic orbitals.
The carbon atoms in benzene are in a state of sp 2 hybridization. Each "C" atom forms three σ bonds (two with carbon atoms and one with a hydrogen atom). All σ bonds are in the same plane:
Each carbon atom has one p-electron, which does not participate in hybridization. The unhybridized p-orbitals of carbon atoms are in a plane perpendicular to the plane of σ bonds. Each p-cloud overlaps with two neighboring p-clouds, and as a result a single conjugated π-system is formed (remember the effect of conjugation of p-electrons in the 1,3-butadiene molecule, discussed in the topic “Diene hydrocarbons”):
The combination of six σ-bonds with a single π-system is called aromatic connection.
A ring of six carbon atoms linked by an aromatic bond is called benzene ring or benzene ring.
In accordance with modern ideas about electronic structure benzene molecule C 6 H 6 is depicted as follows:

Physical properties of benzene
Benzene under normal conditions is a colorless liquid; t o pl = 5.5 o C; t o kip. = 80 o C; has a characteristic odor; does not mix with water, good solvent, highly toxic.
Chemical properties of benzene
The aromatic bond determines the chemical properties of benzene and other aromatic hydrocarbons.
The 6π-electron system is more stable than ordinary two-electron π-bonds. Therefore, addition reactions are less typical for aromatic hydrocarbons than for unsaturated hydrocarbons. The most characteristic reactions for arenes are substitution reactions.
I. Substitution reactions
1.Halogenation

2. Nitration
The reaction is carried out with a mixture of acids (nitrating mixture): 
3.Sulfonation

4.Alkylation (replacement of the “H” atom with an alkyl group) – Friedel-Crafts reactions, benzene homologues are formed:

Instead of haloalkanes, alkenes can be used (in the presence of a catalyst - AlCl 3 or inorganic acid):

II. Addition reactions
1.Hydrogenation

2.Addition of chlorine

III.Oxidation reactions
1. Combustion
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O
2. Incomplete oxidation (KMnO 4 or K 2 Cr 2 O 7 in an acidic environment). The benzene ring is resistant to oxidizing agents. No reaction occurs.
Obtaining benzene
In industry:
1) oil and coal processing;
2) dehydrogenation of cyclohexane:

3) dehydrocyclization (aromatization) of hexane:

In the laboratory:
Fusion of benzoic acid salts with:

Isomerism and nomenclature of benzene homologues
Any homolog of benzene has a side chain, i.e. alkyl radicals bound to a benzene ring. The first benzene homologue is a benzene ring bonded to a methyl radical: 
Toluene has no isomers, since all positions in the benzene ring are equivalent.
For subsequent homologues of benzene, one type of isomerism is possible - side chain isomerism, which can be of two types:
1) isomerism of the number and structure of substituents;
2) isomerism of the position of substituents.



Physical properties of toluene
Toluene- a colorless liquid with a characteristic odor, insoluble in water, soluble in organic solvents. Toluene is less toxic than benzene.
Chemical properties of toluene
I. Substitution reactions
1.Reactions involving the benzene ring
Methylbenzene enters into all substitution reactions in which benzene is involved, and at the same time exhibits higher reactivity, reactions proceed at a higher rate.
The methyl radical contained in the toluene molecule is a substituent of the kind, therefore, as a result of substitution reactions in the benzene ring, ortho- and para-derivatives of toluene are obtained or, in case of an excess of the reagent, tri-derivatives general formula:

a) halogenation


With further chlorination, dichloromethylbenzene and trichloromethylbenzene can be obtained:

II. Addition reactions
Hydrogenation

III.Oxidation reactions
1.Combustion
C 6 H 5 CH 3 + 9O 2 → 7CO 2 + 4H 2 O
2. Incomplete oxidation
Unlike benzene, its homologues are oxidized by certain oxidizing agents; in this case, the side chain is subject to oxidation, in the case of toluene, the methyl group. Mild oxidizing agents such as MnO 2 oxidize it to an aldehyde group, stronger oxidizing agents (KMnO 4) cause further oxidation to an acid: 
Any homologue of benzene with one side chain is oxidized by a strong oxidizing agent such as KMnO4 into benzoic acid, i.e. the side chain breaks with oxidation of the split-off part to CO 2; For example: 
If there are several side chains, each of them is oxidized to a carboxyl group and as a result polybasic acids are formed, for example:

Obtaining toluene:
In industry:
1) oil and coal processing;
2) dehydrogenation of methylcyclohexane:

3) dehydrocyclization of heptane:
In the laboratory:
1) Friedel-Crafts alkylation;
2) Wurtz-Fittig reaction(reaction of sodium with a mixture of halobenzene and haloalkane).
Aromatic hydrocarbons form an important part of the cyclic series of organic compounds. The simplest representative of such hydrocarbons is benzene. The formula of this substance not only distinguished it from a number of other hydrocarbons, but also gave impetus to the development of a new direction in organic chemistry.
Discovery of aromatic hydrocarbons
Aromatic hydrocarbons were discovered in the early 19th century. At that time the most common fuel for street lighting was illuminating gas. From its condensate, the great English physicist Michael Faraday isolated three grams of an oily substance in 1825, described its properties in detail and named it: carbureted hydrogen. In 1834, the German scientist, chemist Mitscherlich, heated benzoic acid with lime and obtained benzene. The formula for this reaction is presented below:
C6 H5 COOH + CaO fusion of C6 H6 + CaCO3.
At that time, the rare benzoic acid was obtained from the resin of benzoic acid, which can be secreted by some tropical plants. In 1845, a new compound was discovered in coal tar, which was a completely accessible raw material for producing a new substance on an industrial scale. Another source of benzene is petroleum obtained from some fields. To meet the needs of industrial enterprises for benzene, it is also obtained by aromatization of certain groups of acyclic hydrocarbons of oil.
The modern version of the name was proposed by the German scientist Liebig. The root of the word "benzene" should be found in Arabic languages- there it is translated as “incense”.
Physical properties of benzene
Benzene is a colorless liquid with a specific odor. This substance boils at a temperature of 80.1 o C, hardens at 5.5 o C and turns into a white crystalline powder. Benzene practically does not conduct heat and electricity, is poorly soluble in water and well soluble in various oils. The aromatic properties of benzene reflect the essence of its structure internal structure: relatively stable benzene ring and uncertain composition.
Chemical classification of benzene
Benzene and its homologues - toluene and ethylbenzene - are an aromatic series of cyclic hydrocarbons. The structure of each of these substances contains a common structure called a benzene ring. The structure of each of the above substances contains a special cyclic group created by six carbon atoms. It is called the benzene aromatic ring.
History of discovery
The establishment of the internal structure of benzene took several decades. The basic principles of the structure (ring model) were proposed in 1865 by the chemist A. Kekule. As the legend tells, a German scientist saw the formula of this element in a dream. Later, a simplified spelling of the structure of a substance called benzene was proposed. The formula of this substance is a hexagon. The symbols for carbon and hydrogen, which should be located at the corners of the hexagon, are omitted. This produces a simple regular hexagon with alternating single and double lines on the sides. The general formula of benzene is shown in the figure below.

Aromatic hydrocarbons and benzene
The chemical formula of this element suggests that addition reactions are not typical for benzene. For it, as for other elements of the aromatic series, substitution reactions of hydrogen atoms in the benzene ring are typical.
Sulfonation reaction
By ensuring the interaction of concentrated sulfuric acid and benzene, increasing the reaction temperature, benzosulfonic acid and water can be obtained. The structural formula of benzene in this reaction is as follows:

Halogenation reaction
Bromine or chromium reacts with benzene in the presence of a catalyst. This produces halogen derivatives. But the nitration reaction takes place using concentrated nitric acid. The final result of the reaction is a nitrogenous compound:

Using nitriding, a well-known explosive is produced - TNT, or trinitotoluene. Few people know that tol is based on benzene. Many other benzene ring-based nitro compounds can also be used as explosives
Electronic formula of benzene
The standard formula of the benzene ring does not accurately reflect the internal structure of benzene. According to it, benzene must have three localized p-bonds, each of which must interact with two carbon atoms. But, as experience shows, benzene does not have ordinary double bonds. The molecular formula of benzene allows you to see that all the bonds in the benzene ring are equivalent. Each of them has a length of about 0.140 nm, which is intermediate between the length of a standard single bond (0.154 nm) and an ethylene double bond (0.134 nm). The structural formula of benzene, depicted with alternating bonds, is imperfect. A more plausible three-dimensional model of benzene looks like the image below.

Each of the atoms of the benzene ring is in a state of sp 2 hybridization. It spends three valence electrons on the formation of sigma bonds. These electrons cover two neighboring carbohydrate atoms and one hydrogen atom. In this case, both electrons and C-C, H-H bonds are in the same plane.
The fourth valence electron forms a cloud in the shape of a three-dimensional figure eight, located perpendicular to the plane of the benzene ring. Each such electron cloud overlaps above the plane of the benzene ring and directly below it with the clouds of two neighboring carbon atoms.
The density of the n-electron clouds of this substance is evenly distributed between all carbon bonds. In this way, a single ring electron cloud is formed. IN general chemistry This structure is called an aromatic electron sextet.
Equivalence of internal bonds of benzene
It is the equivalence of all the faces of the hexagon that explains the uniformity of aromatic bonds, which determine the characteristic chemical and physical properties that benzene possesses. The formula for the uniform distribution of the n-electron cloud and the equivalence of all its internal connections is shown below.

As you can see, instead of alternating single and double lines, the internal structure is depicted as a circle.
The essence of the internal structure of benzene provides the key to understanding the internal structure of cyclic hydrocarbons and expands the possibilities practical application these substances.
Aromatic hydrocarbons- compounds of carbon and hydrogen, the molecule of which contains a benzene ring. The most important representatives of aromatic hydrocarbons are benzene and its homologues - products of the replacement of one or more hydrogen atoms in a benzene molecule with hydrocarbon residues.

The structure of the benzene molecule
The first aromatic compound, benzene, was discovered in 1825 by M. Faraday. Its molecular formula was established - C6H6. If we compare its composition with the composition of a saturated hydrocarbon containing the same number of carbon atoms - hexane (C 6 H 14), then we can see that benzene contains eight less hydrogen atoms. As is known, the appearance of multiple bonds and cycles leads to a decrease in the number of hydrogen atoms in a hydrocarbon molecule. In 1865, F. Kekule proposed its structural formula as cyclohexanthriene-1,3,5.

Thus, a molecule corresponding to the Kekulé formula contains double bonds, therefore, benzene must be unsaturated, i.e., easily undergo addition reactions: hydrogenation, bromination, hydration, etc.

However, data from numerous experiments have shown that benzene undergoes addition reactions only under harsh conditions(at high temperatures and lighting), resistant to oxidation. The most characteristic reactions for it are substitution reactions Therefore, benzene is closer in character to saturated hydrocarbons.
Trying to explain these discrepancies, many scientists have proposed various options for the structure of benzene. The structure of the benzene molecule was finally confirmed by the reaction of its formation from acetylene. In reality, the carbon-carbon bonds in benzene are equivalent, and their properties are not similar to those of either single or double bonds.
Currently, benzene is denoted either by the Kekule formula or by a hexagon in which a circle is depicted.

So what is special about the structure of benzene?
Based on research data and calculations, it was concluded that all six carbon atoms are in a state of sp 2 hybridization and lie in the same plane. The unhybridized p-orbitals of the carbon atoms that make up the double bonds (Kekule formula) are perpendicular to the plane of the ring and parallel to each other.
They overlap each other, forming a single π-system. Thus, the system of alternating double bonds depicted in Kekulé’s formula is a cyclic system of conjugated, overlapping π bonds. This system consists of two toroidal (donut-like) regions of electron density lying on either side of the benzene ring. Thus, it is more logical to depict benzene as a regular hexagon with a circle in the center (π-system) than as cyclohexanthriene-1,3,5.
The American scientist L. Pauling proposed to represent benzene in the form of two boundary structures that differ in the distribution of electron density and constantly transform into each other:

Bond length measurements confirm this assumption. It was found that all C-C bonds in benzene have the same length (0.139 nm). They are slightly shorter than single C-C bonds (0.154 nm) and longer than double bonds (0.132 nm).
There are also compounds whose molecules contain several cyclic structures, for example:

Isomerism and nomenclature of aromatic hydrocarbons
For benzene homologues isomerism of the position of several substituents is characteristic. The simplest homolog of benzene is toluene(methylbenzene) - has no such isomers; the following homologue is presented as four isomers:

The basis of the name of an aromatic hydrocarbon with small substituents is the word benzene. The atoms in the aromatic ring are numbered, starting from senior deputy to junior:

If the substituents are the same, then numbering is carried out according to the shortcut : for example, substance:

called 1,3-dimethylbenzene, not 1,5-dimethylbenzene.
According to the old nomenclature, positions 2 and 6 are called orthopositions, 4 - para-positions, 3 and 5 - meta-positions.


Physical properties of aromatic hydrocarbons
Benzene and its simplest homologues under normal conditions - very toxic liquids with a characteristic unpleasant odor. They dissolve poorly in water, but well in organic solvents.
Chemical properties of aromatic hydrocarbons

Substitution reactions. Aromatic hydrocarbons undergo substitution reactions.
1. Bromination. When reacting with bromine in the presence of a catalyst, iron (III) bromide, one of the hydrogen atoms in the benzene ring can be replaced by a bromine atom:

2. Nitration of benzene and its homologues. When an aromatic hydrocarbon reacts with nitric acid in the presence of sulfuric acid (a mixture of sulfuric and nitric acids called a nitrating mixture), the hydrogen atom is replaced by a nitro group - NO 2:

By reducing nitrobenzene we obtain aniline- a substance that is used to obtain aniline dyes:

This reaction is named after the Russian chemist Zinin.
Addition reactions. Aromatic compounds can also undergo addition reactions to the benzene ring. In this case, cyclohexane and its derivatives are formed.
1. Hydrogenation. Catalytic hydrogenation of benzene occurs at a higher temperature than the hydrogenation of alkenes:

2. Chlorination. The reaction occurs when illuminated with ultraviolet light and is free radical:

Chemical properties of aromatic hydrocarbons - summary



Benzene homologues
The composition of their molecules corresponds to the formula CnH2n-6. The closest homologues of benzene are:
All benzene homologues following toluene have isomers. Isomerism can be associated both with the number and structure of the substituent (1, 2), and with the position of the substituent in the benzene ring (2, 3, 4). Compounds of the general formula C 8 H 10 :

According to the old nomenclature used to indicate the relative location of two identical or different substituents on the benzene ring, the prefixes are used ortho-(abbreviated o-) - substituents are located on neighboring carbon atoms, meta-(m-) - through one carbon atom and pair-(n-) - substituents opposite each other.
The first members of the homologous series of benzene are liquids with a specific odor. They are lighter than water. They are good solvents. Benzene homologues undergo substitution reactions:
bromination:

nitration:

Toluene is oxidized by permanganate when heated:

Reference material for taking the test:
Mendeleev table
Solubility table
Among the huge arsenal organic matter Several compounds can be identified, the discovery and study of which was accompanied by many years of scientific controversy. Benzene rightfully belongs to them. The structure of benzene in chemistry was finally accepted only at the beginning of the 20th century, while the elemental composition of the substance was determined back in 1825, isolating it from coal tar, which was obtained as a by-product of coking coal.
Benzene, together with toluene, anthracene, phenol, and naphthalene, is currently classified as aromatic hydrocarbons. In our article we will look at what this hydrocarbon is, find out the physical properties, for example, such as solubility, boiling point and density of benzene, and also outline the areas of application of the compound in industry and agriculture.
What are arenas?
The chemistry of organic compounds classifies all known substances into several groups, for example, alkanes, alkynes, alcohols, aldehydes, etc. Home distinctive feature Each class of substances is the presence of certain types of bonds. Molecules of saturated hydrocarbons contain only a sigma bond, substances of the ethylene series contain a double bond, and alkynes contain a triple bond. What class does benzene belong to?
The structure of benzene indicates the presence in its molecule of an aromatic ring called the benzene ring. All organic compounds containing one or more such rings in their molecules are classified as arenes (aromatic hydrocarbons). In addition to benzene, which we are now considering, this group includes a large number of very important substances such as toluene, aniline, phenol and others.
How to solve the problem of the structure of an aromatic hydrocarbon molecule
At first, scientists established it by expressing it with the formula C 6 H 6, according to which the relative molecular weight of benzene is 78. Then several options for structural formulas were proposed, but none of them corresponded to the real physical and chemical properties of benzene observed by chemists in laboratory experiments.
About forty years passed before the German researcher A. Kekule presented his version of the structural formula that the benzene molecule has. It contained three double bonds, indicating a possible unsaturated character chemical properties hydrocarbon. This conflicted with the actually existing nature of the interactions of the compound of the formula C 6 H 6 with other substances, for example, with bromine, nitrate acid, and chlorine.

Only after determining the electronic configuration of the benzene molecule in its structural formula the designation of the benzene nucleus (ring) appeared, and it itself is still used in organic chemistry courses.
Electronic configuration of the C6H6 molecule
What kind spatial structure does it have benzene? The structure of benzene was finally confirmed through two reactions: the trimerization of acetylene to form benzene and its reduction with hydrogen to cyclohexane. It turned out that carbon atoms, connecting with each other, form a flat hexagon and are in a state of sp 2 hybridization, using three of their four valence electrons in connection with other atoms.

The remaining six free p-electrons are located perpendicular to the plane of the molecule. Overlapping with each other, they form a common electron cloud called the benzene nucleus.
The nature of one-and-a-half chemical bonds
It is well known that the physical and chemical properties of compounds depend, first of all, on their internal structure and the types of chemical bonds that arise between atoms. Having examined the electronic structure of benzene, we can come to the conclusion that its molecule has neither single nor double bonds, which can be seen in the Kekulé formula. On the contrary, between the carbon atoms everything chemical bonds are equivalent. Moreover, the common π-electron cloud (of all six C atoms) forms a chemical type of bond called sesquicentral, or aromatic. It is this fact that determines the specific properties of the benzene ring and, as a consequence, the character chemical interaction aromatic hydrocarbons with other substances.

Physical properties
As the temperature decreases, the liquid turns into solid phase, and benzene turns into a white crystalline mass. It melts easily at a temperature of 5.5 °C. Under normal conditions, the substance is a colorless liquid with a peculiar odor. Its boiling point is 80.1 °C.
The density of benzene changes with changes in temperature. The higher the temperature, the lower the density. Let's give a few examples. At a temperature of 10°, the density is 0.8884 g/ml, and at 20° - 0.8786 g/ml. Benzene molecules are non-polar, so the substance is insoluble in water. But the compound itself is good, for example, for fats.

Features of the chemical properties of benzene
It has been experimentally established that the aromatic benzene ring is stable, i.e. characterized by high resistance to tearing. This fact explains the tendency of a substance to undergo substitution-type reactions, for example, with chlorine under normal conditions, with bromine, with nitrate acid in the presence of a catalyst. It should be noted that benzene is highly resistant to oxidizing agents such as potassium permanganate and bromine water. This once again confirms the absence of double bonds in the arene molecule. Severe oxidation, otherwise called combustion, is characteristic of all aromatic hydrocarbons. Since the percentage of carbon in the C 6 H 6 molecule is high, the combustion of benzene is accompanied by a smoky flame with the formation of soot particles. As a result of the reaction, carbon dioxide and water are formed. An interesting question is: can an aromatic hydrocarbon undergo addition reactions? Let's consider it further in more detail.
What does the rupture of the benzene ring lead to?
Let us recall that arene molecules contain a one-and-a-half bond, which arises as a result of the overlap of six p-electrons of carbon atoms. It is the basis of the benzene nucleus. To destroy it and carry out the addition reaction, a number of special conditions are required, for example, light irradiation, high temperature and pressure, and catalysts. A mixture of benzene and chlorine undergoes an addition reaction under the influence of ultraviolet radiation. The product of this interaction will be hexachlorocyclohexane, a toxic crystalline substance used in agriculture as an insecticide. There is no longer a benzene ring in the hexachlorane molecule; six chlorine atoms have been added to the site where it breaks.

Areas of practical application of benzene
In various industries, the substance is widely used as a solvent, as well as a raw material for the further production of varnishes, plastics, dyes, and as an additive to motor fuel. Benzene derivatives and its homologues have an even wider range of applications. For example, nitrobenzene C 6 H 5 NO 2 is the main reagent for the production of aniline. As a result, hexachlorobenzene is obtained with chlorine in the presence of aluminum chloride as a catalyst. It is used for pre-sowing treatment of seeds, and is also used in the woodworking industry to protect wood from pests. Nitration of a benzene homologue (toluene) produces an explosive known as TNT or tol.
In this article, we examined such properties of an aromatic compound as addition and substitution reactions, combustion of benzene, and also identified the areas of its application in industry and agriculture.
Benzene. Formula 1)
Benzene - organic compound C 6 H 6, the simplest aromatic hydrocarbon; mobile colorless volatile liquid with a peculiar mild odor.
- tnl = 5.5°C;
- t kip = 80.1°C;
- density 879.1 kg/m 3 (0.8791 g/cm 3) at 20°C.
With air in a volume concentration of 1.5-8%, benzene forms explosive mixtures. Benzene is mixed in all proportions with ether, gasoline and other organic solvents; 0.054 g of water dissolves in 100 g of benzene at 26°C; with water it forms an azeotropic (constantly boiling) mixture (91.2% benzene by weight) with t kip = 69.25°C.
Story
Benzene was discovered by M. Faraday. (1825), who isolated it from the liquid condensate of illuminating gas; Benzene was obtained in its pure form in 1833 by E. Mitscherlich by dry distillation of the calcium salt of benzoic acid (hence the name).
In 1865, F.A. Kekule proposed a structural formula for benzene corresponding to cyclohexatriene - a closed chain of 6 carbon atoms with alternating single and double bonds. The Kekule formula is quite widely used, although many facts have accumulated indicating that benzene does not have the structure of cyclohexatriene. Thus, it has long been established that ortho-disubstituted benzenes exist only in one form, while the Kekule formula allows for isomerism of such compounds (substituents on carbon atoms connected by a single or double bond). In 1872, Kekule additionally introduced the hypothesis that the bonds in benzene constantly and very quickly move and oscillate. Other formulas for the structure of benzene were proposed, but they did not receive recognition.
Chemical properties

Benzene. Formula (2)
The chemical properties of benzene formally correspond to some extent to formula (1). So, under certain conditions, 3 molecules of chlorine or 3 molecules of hydrogen are added to a benzene molecule; benzene is formed by the condensation of 3 acetylene molecules. However, benzene is characterized mainly not by addition reactions typical of unsaturated compounds, but by electrophilic substitution reactions. In addition, the benzene ring is very resistant to oxidizing agents such as potassium permanganate, which also contradicts the presence of localized double bonds in benzene. Special, so-called The aromatic properties of benzene are explained by the fact that all the bonds in its molecule are aligned, that is, the distances between neighboring carbon atoms are the same and equal to 0.14 nm, the length of a single C-C bond is 0.154 nm and a double C=C bond is 0.132 nm. The benzene molecule has a symmetry axis of six order; Benzene as an aromatic compound is characterized by the presence of a sextet of p-electrons, forming a single closed stable electronic system. However, there is still no generally accepted formula reflecting its structure; formula (2) is often used.
Effect on the body
Benzene can cause acute and chronic poisoning. Penetrates into the body mainly through the respiratory system, but can also be absorbed through intact skin. The maximum permissible concentration of benzene vapor in the air of working premises is 20 mg/m 3 . It is excreted through the lungs and in the urine. Acute poisoning usually occurs during accidents; most of them characteristic features: headache, dizziness, nausea, vomiting, agitation followed by a depressed state, rapid pulse, drop in blood pressure, in severe cases - convulsions, loss of consciousness. Chronic benzene poisoning is manifested by changes in the blood (impaired bone marrow function), dizziness, general weakness, sleep disturbance, and fatigue; in women - menstrual dysfunction. A reliable measure against benzene vapor poisoning is good ventilation of industrial premises.
Treatment for acute poisoning: rest, warmth, bromide drugs, cardiovascular drugs; for chronic poisoning with severe anemia: transfusion of red blood cells, vitamin B12, iron supplements.
Sources
- Omelyanenko L. M., Senkevich N. A., Clinic and prevention of benzene poisoning, M., 1957;
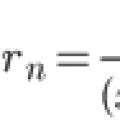 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A