Types of rays. Solar Science
Article navigation:
Radiation and types of radioactive radiation, the composition of radioactive (ionizing) radiation and its main characteristics. The effect of radiation on matter.
What is radiation
First, let's define what radiation is:
In the process of decay of a substance or its synthesis, the elements of an atom (protons, neutrons, electrons, photons) are released, otherwise we can say radiation occurs these elements. Such radiation is called - ionizing radiation or what is more common radioactive radiation, or even simpler radiation . Ionizing radiation also includes x-rays and gamma radiation.
Radiation is the process of emission of charged elementary particles by matter, in the form of electrons, protons, neutrons, helium atoms or photons and muons. The type of radiation depends on which element is emitted.
Ionization is the process of formation of positively or negatively charged ions or free electrons from neutrally charged atoms or molecules.
Radioactive (ionizing) radiation can be divided into several types, depending on the type of elements from which it consists. Different types radiations are caused by different microparticles and therefore have different energetic effects on matter, different abilities to penetrate through it and, as a consequence, different biological effects of radiation.

Alpha, beta and neutron radiation- These are radiations consisting of various particles of atoms.
Gamma and X-rays is the emission of energy.
Alpha radiation

- are emitted: two protons and two neutrons
- penetrating power: low
- irradiation from source: up to 10 cm
- emission speed: 20,000 km/s
- ionization: 30,000 ion pairs per 1 cm of travel
- high
Alpha (α) radiation occurs during the decay of unstable isotopes elements.
Alpha radiation- this is the radiation of heavy, positively charged alpha particles, which are the nuclei of helium atoms (two neutrons and two protons). Alpha particles are emitted during the decay of more complex nuclei, for example, during the decay of atoms of uranium, radium, and thorium.
Alpha particles have a large mass and are emitted at a relatively low speed of an average of 20 thousand km/s, which is approximately 15 times less than the speed of light. Since alpha particles are very heavy, upon contact with a substance, the particles collide with the molecules of this substance, begin to interact with them, losing their energy, and therefore the penetrating ability of these particles is not great and even a simple sheet of paper can hold them back.
However, alpha particles carry a lot of energy and, when interacting with matter, cause significant ionization. And in the cells of a living organism, in addition to ionization, alpha radiation destroys tissue, leading to various damage to living cells.
Of all types of radiation, alpha radiation has the least penetrating power, but the consequences of irradiation of living tissues with this type of radiation are the most severe and significant compared to other types of radiation.
Exposure to alpha radiation can occur when radioactive elements enter the body, for example through air, water or food, or through cuts or wounds. Once in the body, these radioactive elements are carried through the bloodstream throughout the body, accumulate in tissues and organs, exerting a powerful energetic effect on them. Since some types of radioactive isotopes emitting alpha radiation have a long lifespan, when they enter the body, they can cause serious changes in cells and lead to tissue degeneration and mutations.
Radioactive isotopes are actually not eliminated from the body on their own, so once they get inside the body, they will irradiate the tissues from the inside for many years until they lead to serious changes. The human body is not able to neutralize, process, assimilate or utilize most radioactive isotopes that enter the body.
Neutron radiation

- are emitted: neutrons
- penetrating power: high
- irradiation from source: kilometers
- emission speed: 40,000 km/s
- ionization: from 3000 to 5000 ion pairs per 1 cm of run
- biological effects of radiation: high
Neutron radiation- this is man-made radiation arising in various nuclear reactors and during atomic explosions. Also, neutron radiation is emitted by stars in which active thermonuclear reactions occur.
Having no charge, neutron radiation colliding with matter weakly interacts with the elements of atoms at the atomic level, and therefore has high penetrating power. You can stop neutron radiation using materials with a high hydrogen content, for example, a container of water. Also, neutron radiation does not penetrate polyethylene well.
Neutron radiation, when passing through biological tissues, causes serious damage to cells, since it has a significant mass and a higher speed than alpha radiation.
Beta radiation

- are emitted: electrons or positrons
- penetrating power: average
- irradiation from source: up to 20 m
- emission speed: 300,000 km/s
- ionization: from 40 to 150 ion pairs per 1 cm of travel
- biological effects of radiation: average
Beta (β) radiation occurs when one element transforms into another, while the processes occur in the very nucleus of the atom of the substance with a change in the properties of protons and neutrons.
With beta radiation, a neutron is transformed into a proton or a proton into a neutron; during this transformation, an electron or positron (electron antiparticle) is emitted, depending on the type of transformation. The speed of the emitted elements approaches the speed of light and is approximately equal to 300,000 km/s. The elements emitted during this process are called beta particles.
Having an initially high radiation speed and small sizes of emitted elements, beta radiation has a higher penetrating ability than alpha radiation, but has hundreds of times less ability to ionize matter compared to alpha radiation.
Beta radiation easily penetrates through clothing and partially through living tissue, but when passing through denser structures of matter, for example, through metal, it begins to interact with it more intensely and loses most of its energy, transferring it to the elements of the substance. A metal sheet of a few millimeters can completely stop beta radiation.
If alpha radiation poses a danger only in direct contact with a radioactive isotope, then beta radiation, depending on its intensity, can already cause significant harm to a living organism at a distance of several tens of meters from the radiation source.
If a radioactive isotope emitting beta radiation enters a living organism, it accumulates in tissues and organs, exerting an energetic effect on them, leading to changes in the structure of the tissue and, over time, causing significant damage.
Some radioactive isotopes with beta radiation have a long decay period, that is, once they enter the body, they will irradiate it for years until they lead to tissue degeneration and, as a result, cancer.
Gamma radiation

- are emitted: energy in the form of photons
- penetrating power: high
- irradiation from source: up to hundreds of meters
- emission speed: 300,000 km/s
- ionization:
- biological effects of radiation: low
Gamma (γ) radiation is energetic electromagnetic radiation in the form of photons.
Gamma radiation accompanies the process of decay of atoms of matter and manifests itself in the form of emitted electromagnetic energy in the form of photons, released when the energy state of the atomic nucleus changes. Gamma rays are emitted from the nucleus at the speed of light.
When the radioactive decay of an atom occurs, other substances are formed from one substance. Atom again formed substances are in an energetically unstable (excited) state. By influencing each other, neutrons and protons in the nucleus come to a state where the interaction forces are balanced, and excess energy is emitted by the atom in the form of gamma radiation
Gamma radiation has a high penetrating ability and easily penetrates clothing, living tissue, and a little more difficult through dense structures of substances such as metal. To stop gamma radiation, a significant thickness of steel or concrete will be required. But at the same time, gamma radiation has a hundred times weaker effect on matter than beta radiation and tens of thousands of times weaker than alpha radiation.
The main danger of gamma radiation is its ability to travel significant distances and affect living organisms several hundred meters from the source of gamma radiation.
X-ray radiation
- are emitted: energy in the form of photons
- penetrating power: high
- irradiation from source: up to hundreds of meters
- emission speed: 300,000 km/s
- ionization: from 3 to 5 pairs of ions per 1 cm of travel
- biological effects of radiation: low
X-ray radiation- this is energetic electromagnetic radiation in the form of photons that arise when an electron inside an atom moves from one orbit to another.
X-ray radiation is similar in effect to gamma radiation, but has less penetrating power because it has a longer wavelength.
Having considered different kinds radioactive radiation, it is clear that the concept of radiation includes completely different types of radiation that have different effects on matter and living tissues, from direct bombardment elementary particles(alpha, beta and neutron radiation) to energy effects in the form of gamma and x-ray healing.
Each of the radiations discussed is dangerous!
Comparative table with characteristics of different types of radiation
| characteristic | Type of radiation | ||||
| Alpha radiation | Neutron radiation | Beta radiation | Gamma radiation | X-ray radiation | |
| are emitted | two protons and two neutrons | neutrons | electrons or positrons | energy in the form of photons | energy in the form of photons |
| penetrating power | low | high | average | high | high |
| exposure from source | up to 10 cm | kilometers | up to 20 m | hundreds of meters | hundreds of meters |
| radiation speed | 20,000 km/s | 40,000 km/s | 300,000 km/s | 300,000 km/s | 300,000 km/s |
| ionization, steam per 1 cm of travel | 30 000 | from 3000 to 5000 | from 40 to 150 | from 3 to 5 | from 3 to 5 |
| biological effects of radiation | high | high | average | low | low |
As can be seen from the table, depending on the type of radiation, radiation at the same intensity, for example 0.1 Roentgen, will have a different destructive effect on the cells of a living organism. To take this difference into account, a coefficient k was introduced, reflecting the degree of exposure to radioactive radiation on living objects.
| Factor k | |
| Type of radiation and energy range | Weight multiplier |
| Photons all energies (gamma radiation) | 1 |
| Electrons and muons all energies (beta radiation) | 1 |
| Neutrons with energy < 10 КэВ (нейтронное излучение) | 5 |
| Neutrons from 10 to 100 KeV (neutron radiation) | 10 |
| Neutrons from 100 KeV to 2 MeV (neutron radiation) | 20 |
| Neutrons from 2 MeV to 20 MeV (neutron radiation) | 10 |
| Neutrons> 20 MeV (neutron radiation) | 5 |
| Protons with energies > 2 MeV (except for recoil protons) | 5 |
| Alpha particles, fission fragments and other heavy nuclei (alpha radiation) | 20 |
The higher the “k coefficient”, the more dangerous the effect of a certain type of radiation is on the tissues of a living organism.
Video:
Introduction
Ionizing radiation, if we talk about it in general view, are various types of microparticles and physical fields capable of ionizing matter. The main types of ionizing radiation are electromagnetic radiation (X-ray and gamma radiation), as well as streams of charged particles - alpha particles and beta particles, which arise when nuclear explosion. Protection from damaging factors is the basis of the country's civil defense. Let's consider the main types of ionizing radiation.
Types of radiation
Alpha radiation
Alpha radiation is a stream of positively charged particles formed by 2 protons and 2 neutrons. The particle is identical to the nucleus of the helium-4 atom (4He2+). Formed during alpha decay of nuclei. Alpha radiation was first discovered by E. Rutherford. Studying radioactive elements, in particular studying such radioactive elements as uranium, radium and actinium, E. Rutherford came to the conclusion that all radioactive elements emit alpha and beta rays. And, more importantly, the radioactivity of any radioactive element decreases after a certain specific period of time. The source of alpha radiation is radioactive elements. Unlike other types of ionizing radiation, alpha radiation is the most harmless. It is dangerous only when such a substance enters the body (inhalation, eating, drinking, rubbing, etc.), since the range of an alpha particle, for example with an energy of 5 MeV, in the air is 3.7 cm, and in biological tissue 0. 05 mm. Alpha radiation from a radionuclide that enters the body causes truly terrible destruction, because the quality factor of alpha radiation with energy less than 10 MeV is 20 mm. and energy losses occur in a very thin layer of biological tissue. It practically burns him. When alpha particles are absorbed by living organisms, mutagenic (factors that cause mutation), carcinogenic (substances or a physical agent (radiation) that can cause the development of malignant tumors) and other negative effects may occur. Penetrating ability of A.-i. small because held up by a sheet of paper.
Beta radiation
Beta particle (beta particle), a charged particle emitted by beta decay. The stream of beta particles is called beta rays or beta radiation.
Negatively charged beta particles are electrons (b-), positively charged beta particles are positrons (b+).
The energies of beta particles are distributed continuously from zero to some maximum energy, depending on the decaying isotope; this maximum energy ranges from 2.5 keV (for rhenium-187) to tens of MeV (for short-lived nuclei far from the beta stability line).
Beta rays are deviated from the straight direction under the influence of electric and magnetic fields. The speed of particles in beta rays is close to the speed of light.
Beta rays are capable of ionizing gases, causing chemical reactions, luminescence, and affecting photographic plates.
Significant doses of external beta radiation can cause radiation burns to the skin and lead to radiation sickness. Even more dangerous is internal radiation from beta-active radionuclides that enter the body. Beta radiation has significantly less penetrating power than gamma radiation (however, an order of magnitude greater than alpha radiation). A layer of any substance with surface density of the order of 1 g/cm2 (for example, a few millimeters of aluminum or several meters of air) almost completely absorbs beta particles with an energy of about 1 MeV.
Gamma radiation
Gamma radiation is a type of electromagnetic radiation with an extremely short wavelength -< 5Ч10-3 нм и вследствие этого ярко выраженными корпускулярными и слабо выраженными волновыми свойствами. Гамма-квантами являются фотоны высокой энергии. Обычно считается, что энергии квантов гамма-излучения превышают 105 эВ, хотя резкая граница между гамма- и рентгеновским излучением не определена. На шкале электромагнитных волн гамма-излучение граничит с рентгеновским излучением, занимая диапазон более высоких частот и энергий. В области 1-100 кэВ гамма-излучение и рентгеновское излучение различаются только по источнику: если квант излучается в ядерном переходе, то его принято относить к гамма-излучению, если при взаимодействиях электронов или при переходах в атомной электронной оболочке-то к рентгеновскому излучению. Очевидно, физически кванты электромагнитного излучения с одинаковой энергией не отличаются, поэтому такое разделение условно.
Gamma radiation is emitted during transitions between excited states atomic nuclei(the energies of such gamma rays range from ~1 keV to tens of MeV), during nuclear reactions (for example, during the annihilation of an electron and a positron, the decay of a neutral pion, etc.), as well as during the deflection of energetic charged particles in magnetic and electric fields(see Synchrotron radiation).
Gamma rays, unlike b-rays and b-rays, are not deflected by electrical and magnetic fields and are characterized by greater penetrating ability with equal energies and other equal conditions. Gamma rays cause the ionization of atoms of a substance. The main processes that occur when gamma radiation passes through matter:
Photoelectric effect (a gamma quantum is absorbed by an electron of the atomic shell, transferring all the energy to it and ionizing the atom).
Compton scattering (a gamma quantum is scattered by an electron, transferring part of its energy to it).
The birth of electron-positron pairs (in the field of the nucleus, a gamma quantum with an energy of at least 2mec2 = 1.022 MeV is converted into an electron and a positron).
Photonuclear processes (at energies above several tens of MeV, a gamma quantum is capable of knocking nucleons out of the nucleus).
Gamma rays, like any other photons, can be polarized.
Irradiation with gamma quanta, depending on the dose and duration, can cause chronic and acute radiation sickness. Stochastic effects of radiation include various types of cancer. At the same time, gamma irradiation suppresses the growth of cancer and other rapidly dividing cells. Gamma radiation is a mutagenic and teratogenic factor.
A layer of substance can serve as protection against gamma radiation. The effectiveness of protection (that is, the probability of absorption of a gamma quantum when passing through it) increases with increasing thickness of the layer, density of the substance and the content of heavy nuclei in it (lead, tungsten, depleted uranium, etc.).
A person is constantly under the influence of various external factors. Some of them are visible, such as weather conditions, and the extent of their impact can be controlled. Others are not visible to the human eye and are called radiations. Everyone should know the types of radiation, their role and applications.
Humans can encounter some types of radiation everywhere. A prime example is radio waves. They are vibrations of an electromagnetic nature that can be distributed in space at the speed of light. Such waves carry energy from generators.
Radio wave sources can be divided into two groups.
- Natural, these include lightning and astronomical units.
- Artificial, that is, created by man. They include alternating current emitters. These can be radio communication devices, broadcasting devices, computers and navigation systems.
Human skin is capable of depositing this type of waves on its surface, so there are a number of negative consequences of their impact on humans. Radio wave radiation can slow down the activity of brain structures and also cause mutations at the gene level.
For persons who have a pacemaker, such exposure is fatal. These devices have a clear maximum permissible radiation level; rising above it introduces an imbalance in the operation of the stimulator system and leads to its breakdown.
 All the effects of radio waves on the body have been studied only in animals; there is no direct evidence of their negative effect on humans, but scientists are still looking for ways to protect themselves. As such effective ways Not yet. The only thing we can advise is to stay away from dangerous devices. Since household appliances connected to the network also create a radio wave field around themselves, it is simply necessary to turn off the power to devices that a person is not currently using.
All the effects of radio waves on the body have been studied only in animals; there is no direct evidence of their negative effect on humans, but scientists are still looking for ways to protect themselves. As such effective ways Not yet. The only thing we can advise is to stay away from dangerous devices. Since household appliances connected to the network also create a radio wave field around themselves, it is simply necessary to turn off the power to devices that a person is not currently using.
Infrared spectrum radiation
All types of radiation are interconnected in one way or another. Some of them are visible to the human eye. Infrared radiation is adjacent to the part of the spectrum that the human eye can detect. It not only illuminates the surface, but can also heat it.
The main natural source of infrared rays is the sun. Man has created artificial emitters, through which the necessary thermal effect is achieved.
Now we need to figure out how useful or harmful this type of radiation is for humans. Almost all long-wave radiation of the infrared spectrum is absorbed by the upper layers of the skin, so it is not only safe, but can also improve immunity and enhance regenerative processes in tissues.
As for short waves, they can go deep into tissues and cause overheating of organs. The so-called heat stroke is a consequence of exposure to short infrared waves. The symptoms of this pathology are known to almost everyone:
- the appearance of dizziness in the head;
- feeling of nausea;
- increase in heart rate;
- visual impairment characterized by darkening of the eyes.
How to protect yourself from dangerous influences? It is necessary to observe safety precautions, using heat-protective clothing and screens. The use of short-wave heaters must be strictly dosed; the heating element must be covered with heat-insulating material, with the help of which radiation of soft long waves is achieved.
If you think about it, all types of radiation can penetrate tissue. But it was X-ray radiation that made it possible to use this property in practice in medicine.
If we compare X-ray rays with light rays, the former are very long, which allows them to penetrate even opaque materials. Such rays are unable to be reflected or refracted. This type of spectrum has a soft and hard component. Soft consists of long waves that can be completely absorbed by human tissue. Thus, constant exposure to long waves leads to cell damage and DNA mutation.

There are a number of structures that are not able to transmit x-rays through themselves. These include, for example, bone tissue and metals. Based on this, photographs of human bones are taken to diagnose their integrity.
Currently, devices have been created that make it possible not only to take a fixed photograph, for example, of a limb, but also to observe the changes occurring in it “online”. These devices help the doctor perform surgery on the bones under visual control, without making wide traumatic incisions. Using such devices, it is possible to study the biomechanics of joints.
As for the negative effects of X-rays, prolonged contact with them can lead to the development of radiation sickness, which manifests itself in a number of signs:
- neurological disorders;
- dermatitis;
- decreased immunity;
- inhibition of normal hematopoiesis;
- development of oncological pathology;
- infertility.
To protect yourself from dire consequences, when coming into contact with this type of radiation, you need to use shields and linings made of materials that do not transmit rays.
People are accustomed to simply calling this type of rays light. This type of radiation can be absorbed by the object of influence, partially passing through it and partially being reflected. Such properties are widely used in science and technology, especially in the manufacture of optical instruments.
All sources of optical radiation are divided into several groups.
- Thermal, having a continuous spectrum. Heat is released in them due to current or combustion process. These can be electric and halogen incandescent lamps, as well as pyrotechnic products and electric lighting devices.
- Luminescent, containing gases excited by streams of photons. Such sources are energy-saving devices and cathodoluminescent devices. As for radio- and chemiluminescent sources, the flows in them are excited due to radioactive decay products and chemical reactions respectively.
- Plasma, whose characteristics depend on the temperature and pressure of the plasma formed in them. These can be gas-discharge, mercury tube and xenon lamps. Spectral sources, as well as pulsed devices, are no exception.
Optical radiation acts on the human body in combination with ultraviolet radiation, which provokes the production of melanin in the skin. Thus, positive effect lasts until a threshold exposure value is reached, beyond which there is a risk of burns and skin cancer.
The most famous and widely used radiation, the effects of which can be found everywhere, is ultraviolet radiation. This radiation has two spectra, one of which reaches the earth and participates in all processes on earth. The second is retained by the ozone layer and does not pass through it. The ozone layer neutralizes this spectrum, thereby performing a protective role. The destruction of the ozone layer is dangerous due to the penetration of harmful rays onto the surface of the earth.
The natural source of this type of radiation is the Sun. A huge number of artificial sources have been invented:
- Erythema lamps that activate the production of vitamin D in the layers of the skin and help treat rickets.
- Solariums not only allow you to sunbathe, but also have a healing effect for people with pathologies caused by a lack of sunlight.
- Laser emitters used in biotechnology, medicine and electronics.
As for the effect on the human body, it is twofold. On the one hand, a lack of ultraviolet radiation can cause various diseases. A dosed load of such radiation helps the immune system, muscle and lung function, and also prevents hypoxia.
All types of influences are divided into four groups:
- ability to kill bacteria;
- relieving inflammation;
- restoration of damaged tissues;
- reduction of pain.
 The negative effects of ultraviolet radiation include the ability to provoke skin cancer with prolonged exposure. Melanoma of the skin is an extremely malignant type of tumor. Such a diagnosis almost 100 percent means impending death.
The negative effects of ultraviolet radiation include the ability to provoke skin cancer with prolonged exposure. Melanoma of the skin is an extremely malignant type of tumor. Such a diagnosis almost 100 percent means impending death.
As for the organ of vision, excessive exposure to ultraviolet rays damages the retina, cornea and membranes of the eye. Thus, this type of radiation should be used in moderation. If, under certain circumstances, you have to be in contact with a source of ultraviolet rays for a long time, then it is necessary to protect your eyes with glasses and your skin with special creams or clothing.
These are the so-called cosmic rays, which carry the nuclei of atoms of radioactive substances and elements. The gamma radiation flux has very high energy and is able to quickly penetrate the body's cells, ionizing their contents. Destroyed cellular elements act as poisons, decomposing and poisoning the entire body. The cell nucleus is necessarily involved in the process, which leads to mutations in the genome. Healthy cells are destroyed, and in their place mutant cells are formed that are unable to fully provide the body with everything it needs.
This radiation is dangerous because a person does not feel it at all. The consequences of exposure do not appear immediately, but have a long-term effect. The cells of the hematopoietic system, hair, genital organs and lymphoid system are primarily affected.
Radiation is very dangerous for the development of radiation sickness, but even this spectrum has found useful applications:

- it is used to sterilize products, equipment and instruments for medical purposes;
- measuring the depth of underground wells;
- measuring the path length of spacecraft;
- impact on plants in order to identify productive varieties;
- In medicine, such radiation is used for radiation therapy in the treatment of oncology.
In conclusion, it must be said that all types of rays are successfully used by humans and are necessary. Thanks to them, plants, animals and people exist. Protection against overexposure should be a priority when working.
On the eve of summer, I already want to talk about the sun. That's why we have a new regular column dedicated to SPF, where we will tell you all about radiation and how to “get your dose” of vitamin D without danger to your health.
Grade
Let's start off with? that almost everyone knows that this is good. But what is it? Maybe it's not really so scary? Sun Protection Factor is a sun protection factor. It refers to the ability of cosmetics to increase the time of safe exposure to the open sun. The index can be from 2 to 100 units.
Types of sun rays
I don’t want to overload you with complex classifications, but this is what helps us understand. There are three types of rays:
- UVC. They do not reach the surface of the earth.
- UVA. Penetrates into the upper layers of the skin. As a result of their influence, we get a tan due to an increase in the concentration of melanin. There are also back side, because this way you can get burns of varying degrees and develop skin cancer. These rays are especially active from late March to October. They have a cumulative effect.
- UVB. They penetrate not only into the upper, but also into the deep layers of the skin. Provokes photoaging (changes in skin condition).
In moderate doses, ultraviolet light normalizes work immune system, activates the production of vitamin D and is one of the best antidepressants.
If your product indicates combined protection (UVA/UVB), this is a great option. But often manufacturers may indicate other options: UVB/UVC. At the same time, it is already clear that the latest radiation is not scary for us. After all, they do not reach the surface of the earth.

Do you need sun protection all year round?
Let's start with the fact that in the spring our body begins to produce melanin itself. Therefore, it is important to start not with the selection of a protective agent, but with, including. If you have a rough layer, melanin will simply get stuck between the scales and form pigmentation.
UVA rays are active at any time of the day and year. We receive almost 50% of the annual dose of rays outside of the summer.
Should I use protection all year round? It all depends on where you live. If in warm regions - definitely yes. For ordinary residents of the metropolis, the rules are simple. You really should always apply such products, but not every day.
- In winter, many people like to go skiing or fishing. The radiation level is very high. It is worth taking protection of at least SPF 30.
- Use the products in the spring. After all, the sun is already beginning to actively act, and we love open terraces and long walks outside.
- Apply sunscreen products during the most dangerous times, from 11 a.m. to 4 p.m.
- Cream with SPF is a godsend in the summer.
On cloudy days, the skin also needs protection, because clouds block only 20% of the rays.
The sun helps synthesize vitamin D, so you shouldn’t deny yourself sunbathing, but you need to know when to stop and use products that will help you avoid photoaging and maintain youth. Soon we will tell you how to choose your type.
Photo by on , Photo by
A person cannot live without the sun's rays. The sun gives us joy and helps us stay healthy. Sun rays affect the production of serotonin, which improves mood and performance. They are necessary for the synthesis of vitamin D3, which is important for bones, without which calcium cannot be absorbed in the body.
As a matter of fact, what is considered the “sun” in our minds is actually just not the largest part of it. The human eye can detect only 40% of the sun's rays. The "invisible" Sun is infrared radiation(50%) and ultraviolet (10%).
Types of sun rays:
1. Ultraviolet (UVC, UVB, UVA)
I) UVC - do not reach the Earth’s surface and are completely absorbed by the upper layers of the atmosphere.
II) UVB - do not pass beyond the epidermis, causing a lasting tan.
III) UVA - penetrate the dermis, causing an “instant tan” that appears immediately after exposure to the sun and quickly disappears.
2. Infrared (IR-A, IR-B, IR-C) - thermal radiation from the Sun. IR-A rays are able to penetrate the hypodermis and subcutaneous fat.
Do you remember the rhyme about “Every hunter wants to know where the pheasant sits”? Violet (“pheasant”) is the last visible part of the solar spectrum, followed by ultraviolet. Red (“every”) is the first color of the solar spectrum accessible to our vision, preceded by invisible infrared rays.
Different types of sunlight differ from each other in an important physical characteristic - wavelength, which determines their properties.
- UVB rays are practically unable to penetrate ordinary glass. UVA and IR rays penetrate glass easily. Therefore, sitting near a closed window on a hot day cannot get a tan, but you can get heatstroke.
- Infrared rays are unable to penetrate water. 60% of UVB and 85% of UVA rays penetrate to a sufficient depth. Therefore, when we are in a pond, we do not feel the heat, but we can get a sunburn.
Doctors do not recommend staying in the sun for a long time without using solar cosmetics. It is needed not only during a trip to the sea or an excursion in the desert, but also when you are simply in the fresh air for a long time: working in the garden, taking a walk, skiing or cycling. Solar cosmetics will save you from troubles that can come from the sun's rays.
UVB rays can cause burns and pigment spots on the skin. UVA rays damage collagen and elastin fibers, causing the skin to lose firmness and elasticity.
Infrared A-rays have long been considered harmless. However, research conducted at the University of Dusseldorf in 2003 showed that IRA rays, when exposed to human skin, lead to the formation of free radicals that destroy collagen fibers, leading to premature aging. Ladival was the first to use a patented formula with antioxidants in solar cosmetics to protect against the harmful effects of IRA rays. Its effectiveness has been clinically proven.
5 facts about the Sun:
1. The word "Sun" in English language is an exception: it has the form of a personal pronoun and belongs to the masculine gender - “He”.
2. Lack of sunlight can cause a mental disorder - winter depression (Seasonal Affective Disorder). Its symptoms are drowsiness, lethargy, irritability, a feeling of hopelessness, and anxiety.
3. The mass of the Sun is 99.85% of the mass solar system. The share of its remaining objects accounts for only 0.15%.
4. About 1 million planets the size of Earth could fit inside the Sun.
5. The force of gravity on the Sun is 28 times greater than the force of gravity on Earth: a person on Earth weighs 60 kilograms on the Sun would weigh 1680 kilograms.
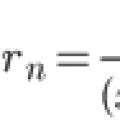 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A