Which process is called internal friction or viscosity. The phenomenon of internal friction (viscosity)
Ideal liquid, i.e. a fluid moving without friction is an abstract concept. All real liquids and gases exhibit viscosity or internal friction to a greater or lesser extent. Viscosity (internal friction), along with diffusion and thermal conductivity, is a transport phenomenon and is observed only in moving liquids and gases. Viscosity is manifested in the fact that the movement that occurs in a liquid or gas, after the cessation of the causes that caused it, gradually ceases.
Viscosity(internal friction) is one of the transfer phenomena, the property of fluid bodies (liquids and gases) to resist the movement of one part of them relative to another. As a result, the energy spent on this movement is dissipated in the form of heat.
The mechanism of internal friction in liquids and gases is that chaotically moving molecules carry impulse from one layer to another, which leads to equalization of velocities - this is described by the introduction of a friction force. Viscosity solids has a number of specific features and is usually considered separately.
In liquids, where the distances between molecules are much smaller than in gases, viscosity is primarily due to intermolecular interactions, which limit the mobility of molecules. In a liquid, a molecule can penetrate into an adjacent layer only if a cavity is formed in it, sufficient for the molecule to jump there. The so-called activation energy of viscous flow is consumed to form a cavity (to “loose” the liquid). The activation energy decreases with increasing temperature and decreasing pressure. This is one of the reasons for the sharp decrease in the viscosity of liquids with increasing temperature and its increase at high pressures. When the pressure increases to several thousand atmospheres, the viscosity increases tens and hundreds of times. A rigorous theory of the viscosity of liquids, due to the insufficient development of the theory of the liquid state, has not yet been created.
The viscosity of individual classes of liquids and solutions depends on temperature, pressure and chemical composition.
The viscosity of liquids depends on the chemical structure of their molecules. In a series of similar chemical compounds (saturated hydrocarbons, alcohols, organic acids, etc.), viscosity changes naturally - it increases with increasing molecular weight. The high viscosity of lubricating oils is explained by the presence of cycles in their molecules. Two liquids of different viscosities that do not react with each other when mixed have an average viscosity in the mixture. If, upon mixing, it forms chemical compound, then the viscosity of the mixture can be tens of times greater than the viscosity of the original liquids.
Occurrence in liquids ( dispersed systems or polymer solutions) spatial structures, formed by the adhesion of particles or macromolecules, causes a sharp increase in viscosity. When a “structured” fluid flows, the work of an external force is spent not only on overcoming viscosity, but also on destroying the structure.
In gases, the distances between molecules are significantly greater than the radius of action of molecular forces, therefore the viscosity of gases is determined mainly by molecular motion. Between layers of gas moving relative to each other, there is a constant exchange of molecules due to their continuous chaotic (thermal) movement. The transition of molecules from one layer to the adjacent one, moving at a different speed, leads to the transfer of a certain momentum from layer to layer. As a result, the slow layers speed up and the faster layers slow down. Work done by external force F, which balances the viscous resistance and maintains a steady flow, is completely converted into heat. The viscosity of a gas does not depend on its density (pressure), since when the gas is compressed, the total number of molecules moving from layer to layer increases, but each molecule penetrates less deeply into the adjacent layer and transfers less momentum (Maxwell’s law).
Viscosity is an important physical and chemical characteristic of substances. The viscosity value must be taken into account when pumping liquids and gases through pipes (oil pipelines, gas pipelines). The viscosity of molten slag is very significant in blast furnace and open-hearth processes. The viscosity of molten glass determines the process of its production. In many cases, viscosity is used to judge the readiness or quality of products or semi-products of production, since viscosity is closely related to the structure of the substance and reflects the physical and chemical changes in the material that occur during technological processes. The viscosity of oils is great importance for calculating the lubrication of machines and mechanisms, etc.
The device for measuring viscosity is called viscometer.
Viscosity coefficient .
Viscosity is one of the most important phenomena observed during the movement of a real fluid.
All real liquids (and gases) exhibit viscosity or internal friction to one degree or another. When a real fluid flows between its layers, friction forces arise. These forces are called forces of internal friction or viscosity.
Viscosity is the friction between layers of liquid (or gas) moving relative to each other.
The forces of viscosity (internal friction) are directed tangentially to the contacting layers of liquid and counteract the movement of these layers relative to each other. They decelerate the faster layer and speed up the slower layer. There are two main reasons for viscosity:
Firstly, interaction forces between molecules of adjacent layers moving at different speeds;
Secondly, the transition of molecules from layer to layer, and the associated transfer of momentum.
Due to these reasons, the layers interact with each other, the slow layer accelerates, the fast layer slows down. In liquids the first reason is more clearly expressed, in gases the second.
To clarify the patterns that govern the forces of internal friction, consider the following experiment. Let's take two horizontal plates with a layer of liquid between them (Fig. 9). We set the upper plate in motion at a constant speed  . To do this, force must be applied to the plate
. To do this, force must be applied to the plate  to overcome friction force
to overcome friction force  , acting on the plate as it moves in the liquid. The layer of liquid adjacent directly to the top plate, due to wetting, adheres to the plate and moves with it. The layer of liquid adhering to the bottom plate is held at rest with it,
, acting on the plate as it moves in the liquid. The layer of liquid adjacent directly to the top plate, due to wetting, adheres to the plate and moves with it. The layer of liquid adhering to the bottom plate is held at rest with it,  . The intermediate layers move in such a way that each upper one has a speed greater than the one lying underneath it. The arrows in Fig. 9 show the “velocity profile” of the flow. Along the axis perpendicular to the vector
. The intermediate layers move in such a way that each upper one has a speed greater than the one lying underneath it. The arrows in Fig. 9 show the “velocity profile” of the flow. Along the axis perpendicular to the vector  , the speed increases. Speed measurement is characterized by the value
, the speed increases. Speed measurement is characterized by the value  .
.
Magnitude  shows what measurement of speed is per unit length along the direction of change of speed, i.e.
shows what measurement of speed is per unit length along the direction of change of speed, i.e.  determines the rate of change in speed and direction perpendicular to the speed itself. The friction between the layers depends on this value. Magnitude
determines the rate of change in speed and direction perpendicular to the speed itself. The friction between the layers depends on this value. Magnitude  measured in
measured in  .
.
Newton discovered that the frictional force between two layers of liquid is directly proportional to the area of contact between the layers  and size
and size  :
:


 .
(13)
.
(13)
Formula (13) is called Newton's formula for viscous friction. Proportionality factor  called the viscosity coefficient (internal friction). From (13) it is clear that
called the viscosity coefficient (internal friction). From (13) it is clear that

In system  The unit of measure for the viscosity coefficient is
The unit of measure for the viscosity coefficient is
 (pascal - second),
(pascal - second),
in the SGS system, the viscosity coefficient is measured in  (poises), and
(poises), and

Liquids for which Newton’s formula (13) is satisfied are called Newtonian. For such liquids, the viscosity coefficient depends only on temperature. Among biological fluids, Newtonian fluids include blood plasma and lymph. For many real liquids, relation (13) is not strictly satisfied. Such liquids are called non-Newtonian. For them the viscosity coefficient  depends on temperature, pressure and a number of other quantities. These fluids include fluids with large, complex molecules, such as whole blood.
depends on temperature, pressure and a number of other quantities. These fluids include fluids with large, complex molecules, such as whole blood.
Blood viscosity of a healthy person  , with pathology fluctuates, which affects the erythrocyte sedimentation rate. The viscosity of venous blood is greater than that of arterial blood.
, with pathology fluctuates, which affects the erythrocyte sedimentation rate. The viscosity of venous blood is greater than that of arterial blood.
Internal friction I
Internal friction
II
Internal friction
in solids, the property of solids to be irreversibly converted into heat mechanical energy, imparted to the body during its deformation. Voltage is associated with two different groups of phenomena—inelasticity and plastic deformation. Inelasticity is a deviation from the properties of elasticity when a body is deformed under conditions where there is practically no residual deformation. When deforming at a finite speed, a deviation from thermal equilibrium. For example, when bending a uniformly heated thin plate, the material of which expands when heated, the stretched fibers will cool, the compressed fibers will heat up, resulting in a transverse temperature difference, i.e. elastic deformation will cause a violation of thermal equilibrium. Subsequent temperature equalization by thermal conduction is a process accompanied by the irreversible transition of part of the elastic energy into thermal energy. This explains the experimentally observed damping of free bending vibrations of the plate - the so-called Thermoelastic effect. This process of restoring disturbed balance is called relaxation (See Relaxation). During elastic deformation of an alloy with a uniform distribution of atoms of various components, a redistribution of atoms in the substance may occur due to the difference in their sizes. The restoration of the equilibrium distribution of atoms by diffusion (See Diffusion) is also a relaxation process. Manifestations of inelastic, or relaxation, properties, in addition to those mentioned, are elastic aftereffect in pure metals and alloys, elastic hysteresis, etc. The deformation that occurs in an elastic body depends not only on the external mechanical forces applied to it, but also on the temperature of the body, its chemical composition, external magnetic and electric fields (magneto- and electrostriction), grain size, etc. This leads to a variety of relaxation phenomena, each of which makes its own contribution to W. t. If several relaxation processes occur in the body simultaneously, each of which can be characterized by its own relaxation time (See Relaxation) τ i, then the totality of all relaxation times of individual relaxation processes forms the so-called relaxation spectrum of a given material ( rice.
), characterizing a given material under given conditions; Each structural change in the sample changes the relaxation spectrum. The following methods are used for measuring voltage: studying the damping of free vibrations (longitudinal, transverse, torsional, bending); study of the resonance curve for forced oscillations (See Forced oscillations); relative dissipation of elastic energy during one period of oscillation. The study of solid state physics is a new, rapidly developing field of solid state physics and is a source of important information about the processes that occur in solids, in particular in pure metals and alloys that have been subjected to various mechanical and thermal treatments. V. t. during plastic deformation. If the forces acting on a solid body exceed the elastic limit and plastic flow occurs, then we can talk about quasi-viscous resistance to flow (by analogy with a viscous fluid). The mechanism of high stress during plastic deformation differs significantly from the mechanism of high voltage during inelasticity (see Plasticity, Creep).
The difference in energy dissipation mechanisms also determines the difference in viscosity values, which differ by 5-7 orders of magnitude (plastic flow viscosity, reaching values of 10 13 -10 8 n· sec/m 2, is always significantly higher than the viscosity calculated from elastic vibrations and equal to 10 7 -
10 8 n· sec/m 2). As the amplitude of elastic vibrations increases, plastic shears begin to play an increasingly important role in the damping of these vibrations, and the value of viscosity increases, approaching the values of plastic viscosity. Lit.: Novik A.S., Internal friction in metals, in the book: Advances in metal physics. Sat. articles, trans. from English, part 1, M., 1956; Postnikov V.S., Relaxation phenomena in metals and alloys subjected to deformation, “Uspekhi Fizicheskikh Nauk”, 1954, v. 53, v. 1, p. 87; him, Temperature dependence of internal friction of pure metals and alloys, ibid., 1958, vol. 66, century. 1, p. 43.
Big Soviet encyclopedia. - M.: Soviet Encyclopedia. 1969-1978 .
See what “Internal friction” is in other dictionaries:
1) the property of solids to irreversibly absorb mechanical energy received by the body during its deformation. Internal friction manifests itself, for example, in the damping of free vibrations.2) In liquids and gases, the same as viscosity ... Big Encyclopedic Dictionary
INTERNAL FRICTION is the same as viscosity... Modern encyclopedia
In solids, the property of solids is irreversibly converted into mechanical heat. energy imparted to a body during the process of its deformation. V. t. is associated with two different. groups of phenomena of inelasticity and plasticity. deformation. Inelasticity represents... ... Physical encyclopedia- 1) the property of solids to irreversibly convert mechanical energy received by the body during its deformation into heat. Internal friction manifests itself, for example, in the damping of free vibrations. 2) In liquids and gases the same as viscosity. * * *… … encyclopedic Dictionary
Internal friction Internal friction. Conversion of energy into heat under the influence of oscillatory stress of a material. (Source: “Metals and alloys. Directory.” Edited by Yu.P. Solntsev; NPO Professional, NPO Mir and Family; St. Petersburg ... Dictionary of metallurgical terms
Viscosity (internal friction) is a property of solutions that characterizes the resistance to external forces that cause their flow. (See: SP 82 101 98. Preparation and use of construction mortars.)
) mechanical energy imparted to a body during its deformation. Internal friction manifests itself, for example, in the damping of free vibrations. In liquids and gases, a similar process is usually called viscosity. Internal friction in solids is associated with two different groups of phenomena - inelasticity and plastic deformation.
Inelasticity is a deviation from the properties of elasticity when a body is deformed under conditions where there is practically no residual deformation. When deforming at a finite rate, a deviation from thermal equilibrium occurs in the body. For example, when bending a uniformly heated thin plate, the material of which expands when heated, the stretched fibers will cool, the compressed fibers will heat up, resulting in a transverse temperature difference, that is, elastic deformation will cause a violation of thermal equilibrium. Subsequent temperature equalization by thermal conduction is a process accompanied by the irreversible transition of part of the elastic energy into thermal energy. This explains the experimentally observed damping of free bending vibrations of the plate - the so-called thermoelastic effect. This process of restoring disturbed balance is called relaxation.
During elastic deformation of an alloy with a uniform distribution of atoms of various components, a redistribution of atoms in the substance may occur due to the difference in their sizes. The restoration of the equilibrium distribution of atoms by diffusion is also a relaxation process. Manifestations of inelastic, or relaxation, properties are also elastic aftereffects in pure metals and alloys, elastic hysteresis.
The deformation that occurs in an elastic body depends not only on the external mechanical forces applied to it, but also on the temperature of the body, its chemical composition, external magnetic and electric fields (magnetostriction and electrostriction), and grain size. This leads to a variety of relaxation phenomena, each of which makes its own contribution to internal friction. If several relaxation processes occur simultaneously in the body, each of which can be characterized by its own relaxation time, then the totality of all relaxation times of individual relaxation processes forms the so-called relaxation spectrum of a given material; Each structural change in the sample changes the relaxation spectrum.
The following methods are used for measuring internal friction: studying the damping of free vibrations (longitudinal, transverse, torsional, bending); study of the resonance curve for forced oscillations; relative dissipation of elastic energy during one period of oscillation. The study of internal friction of solids is a field of solid state physics and is a source of information about the processes that occur in solids, in particular in pure metals and alloys subjected to mechanical and thermal treatments.
If the forces acting on a solid body exceed the elastic limit and plastic flow occurs, then we can talk about quasi-viscous resistance to flow (by analogy with a viscous fluid). The mechanism of internal friction during plastic deformation differs significantly from the mechanism of internal friction during inelasticity. The difference in energy dissipation mechanisms determines the difference in viscosity values, which differ by 5-7 orders of magnitude. As the amplitude of elastic vibrations increases, plastic shears begin to play a large role in the damping of these vibrations, and the viscosity value increases, approaching the values of plastic viscosity.
Viscosity(internal friction) ( English. viscosity) is one of the transfer phenomena, the property of fluid bodies (liquids and gases) to resist the movement of one part of them relative to another. The mechanism of internal friction in liquids and gases is that chaotically moving molecules transfer momentum from one layer to another, which leads to equalization of velocities - this is described by the introduction of a friction force. The viscosity of solids has a number of specific features and is usually considered separately. The basic law of viscous flow was established by I. Newton (1687): When applied to liquids, viscosity is distinguished:
- Dynamic (absolute) viscosity µ – a force acting on a unit area of a flat surface that moves at a unit speed relative to another flat surface located at a unit distance from the first. In the SI system, dynamic viscosity is expressed as Pa×s(pascal second), non-system unit P (poise).
- Kinematic viscosity ν – dynamic viscosity ratio µ to liquid density ρ .
- ν , m 2 /s – kinematic viscosity;
- μ , Pa×s – dynamic viscosity;
- ρ , kg/m 3 – liquid density.
Viscous friction force
This is the phenomenon of the occurrence of tangential forces that prevent the movement of parts of a liquid or gas relative to each other. Lubrication between two solid bodies replaces dry friction sliding is the sliding friction of layers of liquid or gas relative to each other. The speed of particles in the medium changes smoothly from the speed of one body to the speed of another body.
The force of viscous friction is proportional to the speed of relative motion V bodies, proportional to area S and inversely proportional to the distance between the planes h.
F=-V S / h,The proportionality coefficient, depending on the type of liquid or gas, is called coefficient of dynamic viscosity. The most important thing about the nature of viscous friction forces is that in the presence of any force, no matter how small, the bodies will begin to move, that is, there is no static friction. Qualitatively significant difference in forces viscous friction from dry friction
If a moving body is completely immersed in a viscous medium and the distances from the body to the boundaries of the medium are much greater than the dimensions of the body itself, then in this case we speak of friction or medium resistance. In this case, sections of the medium (liquid or gas) directly adjacent to the moving body move at the same speed as the body itself, and as they move away from the body, the speed of the corresponding sections of the medium decreases, becoming zero at infinity.
The resistance force of the medium depends on:
- its viscosity
- on body shape
- on the speed of movement of the body relative to the medium.
For example, when a ball moves slowly in a viscous fluid, the friction force can be found using the Stokes formula:
F=-6 R V,There is a qualitatively significant difference between the forces of viscous friction and dry friction, among other things, that a body in the presence of only viscous friction and an arbitrarily small external force will necessarily begin to move, that is, for viscous friction there is no static friction, and vice versa - under the influence of only viscous friction, a body that initially moved will never (in within the framework of a macroscopic approximation that neglects Brownian motion) will not stop completely, although the motion will slow down indefinitely.
Gas viscosity
The viscosity of gases (the phenomenon of internal friction) is the appearance of friction forces between layers of gas moving relative to each other in parallel and at different speeds. The viscosity of gases increases with increasing temperature
The interaction of two layers of gas is considered as a process during which momentum is transferred from one layer to another. The frictional force per unit area between two layers of gas, equal to the momentum transferred per second from layer to layer through a unit area, is determined by Newton's law:
τ=-η dν / dz
Where:
dν/dz- velocity gradient in the direction perpendicular to the direction of movement of the gas layers.
The minus sign indicates that the momentum is transferred in the direction of decreasing velocity.
η
- dynamic viscosity.
η= 1 / 3 ρ(ν) λ, where:
ρ
- gas density,
(ν)
- arithmetic average speed of molecules
λ
- the average free path of molecules.
Viscosity of some gases (at 0°C)
Liquid viscosity
Liquid viscosity- this is a property that manifests itself only when a fluid moves, and does not affect fluids at rest. Viscous friction in liquids obeys the law of friction, which is fundamentally different from the law of friction of solids, because depends on the friction area and the speed of fluid movement.
Viscosity– the property of a liquid to resist the relative shear of its layers. Viscosity manifests itself in the fact that with the relative movement of layers of liquid, shear resistance forces arise on the surfaces of their contact, called internal friction forces, or viscous forces. If we consider how the velocities of different layers of liquid are distributed across the cross section of the flow, we can easily notice that the further away from the walls of the flow, the greater the speed of particle movement. At the walls of the flow, the fluid velocity is zero. This is illustrated by a drawing of the so-called jet flow model.

A slowly moving layer of liquid “brakes” an adjacent layer of liquid moving faster, and vice versa, a layer moving at a higher speed drags (pulls) along a layer moving at a lower speed. Internal friction forces appear due to the presence of intermolecular bonds between moving layers. If we select a certain area between adjacent layers of liquid S, then according to Newton's hypothesis:
F=μ S (du / dy),- μ - coefficient of viscous friction;
- S– friction area;
- du/dy- velocity gradient
Magnitude μ in this expression is dynamic viscosity coefficient, equal to:
μ= F / S 1 / du / dy , μ= τ 1/du/dy,- τ – tangential stress in the liquid (depends on the type of liquid).
Physical meaning of the viscous friction coefficient- a number equal to the friction force developing on a unit surface with a unit velocity gradient.
In practice it is more often used kinematic viscosity coefficient, so called because its dimension lacks the designation of force. This coefficient is the ratio of the dynamic coefficient of viscosity of a liquid to its density:
ν= μ / ρ ,Units of viscous friction coefficient:
- N·s/m 2 ;
- kgf s/m 2
- Pz (Poiseuille) 1(Pz)=0.1(N s/m 2).
Fluid Viscosity Property Analysis
For dropping liquids, viscosity depends on temperature t and pressure R, however, the latter dependence appears only with large changes in pressure, on the order of several tens of MPa.
The dependence of the coefficient of dynamic viscosity on temperature is expressed by a formula of the form:
μ t =μ 0 e -k t (T-T 0),- μt - coefficient of dynamic viscosity at a given temperature;
- μ 0 - coefficient of dynamic viscosity at a known temperature;
- T - set temperature;
- T 0 - temperature at which the value is measured μ 0 ;
- e
The dependence of the relative coefficient of dynamic viscosity on pressure is described by the formula:
μ р =μ 0 e -k р (Р-Р 0),- μ R - coefficient of dynamic viscosity at a given pressure,
- μ 0 - coefficient of dynamic viscosity at a known pressure (most often under normal conditions),
- R - set pressure;
- P 0 - pressure at which the value is measured μ 0 ;
- e – the base of the natural logarithm is equal to 2.718282.
The effect of pressure on the viscosity of a liquid appears only at high pressures.
Newtonian and non-Newtonian fluids
Newtonian fluids are those for which the viscosity does not depend on the rate of deformation. In the Navier-Stokes equation for a Newtonian fluid, there is a viscosity law similar to the above (in fact, a generalization of Newton’s law, or Navier’s law).
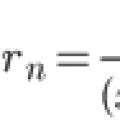 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A