Chemical reactions of halogens with salts. Halogens – Knowledge Hypermarket
From a chemistry textbook, many people know that halogens include chemical elements of the periodic system of Mendeleev from group 17 in the table.
Translated from Greek as birth, origin. Almost all of them are highly active, due to which they react violently with simple substances, with the exception of a few non-metals. What are halogens and what are their properties?
In contact with
List of halogens
Halogens are good oxidizing agents; for this reason, in nature they can only be found in some compounds. The higher the atomic number, the less chemical activity of the elements of this group. The halogen group includes the following elements:
- chlorine (Cl);
- fluorine (F);
- iodine (I);
- bromine (Br);
- astatine (At).
The latter was developed at the Institute of Nuclear Research, which is located in the city of Dubna. Fluorine is a poisonous gas with a pale yellow color. Chlorine is also poisonous. This is a gas that has a rather pungent and unpleasant odor of light green color. Bromine has a reddish-brown color and is a toxic liquid that can even affect the sense of smell. It is very volatile, so it is stored in ampoules. Iodine is a crystalline, easily sublimated, dark purple substance. Astatine is radioactive, crystal color: black with blue, half-life is 8.1 hours.
 The high oxidation activity of halogens decreases from fluorine to iodine. The most active of its brethren is fluorine, which has the ability to react with any metals, forming salts, some of them spontaneously ignite, releasing a huge amount of heat. Without heating, this element reacts with almost all non-metals, reactions are accompanied by the release of a certain amount of heat (exothermic).
The high oxidation activity of halogens decreases from fluorine to iodine. The most active of its brethren is fluorine, which has the ability to react with any metals, forming salts, some of them spontaneously ignite, releasing a huge amount of heat. Without heating, this element reacts with almost all non-metals, reactions are accompanied by the release of a certain amount of heat (exothermic).
Fluorine interacts with inert gases and is irradiated (Xe + F 2 = XeF 2 + 152 kJ). When heated, fluorine affects other halogens, oxidizing them. The formula holds: Hal 2 + F 2 = 2HalF, where Hal = Cl, Br, I, At, in the case when HalF oxidation states of chlorine, bromine, iodine and astatine are equal to + 1.
Fluorine also interacts quite vigorously with complex substances. The consequence is water oxidation. In this case, an explosive reaction occurs, which is briefly written by the formula: 3F 2 + ZH 2 O = OF 2 + 4HF + H 2 O 2.
Chlorine
The activity of free chlorine is slightly less than fluorine, but it also has a good ability to react. This can occur when interacting with many simple substances, with rare exceptions in the form of oxygen, nitrogen, and inert gases. He can react violently with complex substances, creating substitution reactions, the property of adding hydrocarbons is also inherent in chlorine. When heated, bromine or iodine is displaced from compounds with hydrogen or metals.
 This element has a peculiar relationship with hydrogen. At room temperature and without exposure to light, chlorine does not react in any way to this gas, but once it is heated or directed at light, an explosive chain reaction will occur. The formula is given below:
This element has a peculiar relationship with hydrogen. At room temperature and without exposure to light, chlorine does not react in any way to this gas, but once it is heated or directed at light, an explosive chain reaction will occur. The formula is given below:
Cl2+ hν → 2Cl, Cl + H2 → HCl + H, H + Cl2 → HCl + Cl, Cl + H2 → HCl + H, etc.
Photons, when excited, cause the decomposition of Cl 2 molecules into atoms, and a chain reaction occurs, causing the appearance of new particles that initiate the beginning of the next stage. In the history of chemistry this phenomenon has been studied. Russian chemist and Nobel Prize laureate N.N. Semenov. in 1956 he studied the photochemical chain reaction and thereby made a great contribution to science.
Chlorine reacts with many complex substances, these are substitution and addition reactions. It dissolves well in water.
Cl 2 + H 2 O = HCl + HClO - 25 kJ.
With alkalis, when heated, chlorine can disproportionate.
Bromine, iodine and astatine
The chemical activity of bromine is slightly less than that of the above-mentioned fluorine or chlorine, but it is also quite high. Bromine is often used in liquid form. It, like chlorine, dissolves very well in water. A partial reaction occurs with it, allowing one to obtain “bromine water”.
The chemical activity of iodine is noticeably different from other representatives of this series. It almost does not interact with non-metals, but with With metals the reaction occurs very slowly and only when heated. In this case, a large absorption of heat occurs (endothermic reaction), which is highly reversible. Besides Iodine cannot be dissolved in water in any way, this cannot be achieved even with heating, which is why “iodine water” does not exist in nature. Iodine can only be dissolved in iodide solution. In this case, complex anions are formed. In medicine, this compound is called Lugol's solution.
Astatine reacts with metals and hydrogen. In the series of halogens, chemical activity decreases in the direction from fluorine to astatine. Each halogen in the F - At series is capable of displacing subsequent elements from compounds with metals or hydrogen. Astatine is the most passive of these elements. But it is characterized by interaction with metals.
Application
Chemistry is firmly entrenched in our lives, penetrating into all areas. Man has learned to use halogens, as well as its compounds, for his own benefit. The biological significance of halogens is undeniable. Their areas of application are different:
- medicine;
- pharmacology;
- production of various plastics, dyes, etc.;
- Agriculture.
 From the natural compound cryolite, the chemical formula of which is as follows: Na3AlF6, is obtained aluminum. Fluorine compounds are widely used in production toothpastes. Fluoride is known to help prevent caries. Alcohol tincture of iodine is used for disinfection and disinfection of wounds.
From the natural compound cryolite, the chemical formula of which is as follows: Na3AlF6, is obtained aluminum. Fluorine compounds are widely used in production toothpastes. Fluoride is known to help prevent caries. Alcohol tincture of iodine is used for disinfection and disinfection of wounds.
Chlorine has found the most widespread use in our lives. The scope of its application is quite diverse. Examples of using:
- Production of plastics.
- Obtaining hydrochloric acid.
- Production of synthetic fibers, solvents, rubbers, etc.
- Bleaching of fabrics (linen and cotton), paper.
- Disinfection of drinking water. But ozone is increasingly used for this purpose, since the use of chlorine is harmful to the human body.
- Disinfection of premises
It must be remembered that halogens are very toxic substances. This property is especially pronounced in fluorine. Halogens can cause asphyxiation, respiratory irritation and damage biological tissue.
Chlorine vapors can be extremely dangerous, as well as fluorine aerosol, which has a faint odor and can be felt in high concentrations. A person may experience a suffocation effect. When working with such connections, precautions must be taken.
The methods for producing halogens are complex and varied. In industry, this is approached with certain requirements, which are strictly observed.
Chemistry of Elements
Nonmetals of VIIA subgroup
Elements of the VIIA subgroup are typical nonmetals with high
electronegativity, they have a group name - “halogens”.
Main issues covered in the lecture
General characteristics of non-metals of the VIIA subgroup. Electronic structure, the most important characteristics of atoms. The most characteristic ste-
oxidation penalties. Features of the chemistry of halogens.
Simple substances.
Natural compounds.
Halogen compounds
Hydrohalic acids and their salts. Salt and hydrofluoric acid
slots, receipt and application.
Halide complexes.
Binary oxygen compounds of halogens. Instability approx.
Redox properties of simple substances and co-
unities. Disproportionation reactions. Latimer diagrams.
Executor: |
Event No. |
||||||||||||||||

Chemistry of elements of the VIIA subgroup
general characteristics
Manganese |
||||||||
Technetium |
||||||||
VIIA-group is formed by p-elements: fluorine F, chlorine
Cl, bromine Br, iodine I and astatine At.
The general formula for valence electrons is ns 2 np 5.
All elements of group VIIA are typical non-metals.
As can be seen from the distribution |
|||||||
valence electrons |
|||||||
according to orbitals of atoms |
only one electron missing |
||||||
to form a stable eight-electron shell
boxes, that's why they have there is a strong tendency towards
addition of an electron.
All elements easily form simple single-charge
ny anions G – .
In the form of simple anions, elements of group VIIA are found in natural water and in crystals of natural salts, for example, halite NaCl, sylvite KCl, fluorite
CaF2.
General group name of elements VIIA-
group “halogens”, i.e. “giving birth to salts”, is due to the fact that most of their compounds with metals are pre-
is a typical salt (CaF2, NaCl, MgBr2, KI), which
which can be obtained through direct interaction
interaction of metal with halogen. Free halogens are obtained from natural salts, so the name “halogens” is also translated as “born from salts.”
Executor: |
Event No. |
||||||||||||||||

The minimum oxidation state (–1) is the most stable
for all halogens.
Some characteristics of the atoms of Group VIIA elements are given in
The most important characteristics of atoms of elements of group VIIA
Relative- |
Affinity |
||||||
electric |
|||||||
negative |
ionization, |
||||||
ness (according to |
|||||||
Polling) |
|||||||
increase in number |
|||||||
electronic layers; |
|||||||
increase in size |
|||||||
reduction of electrical |
|||||||
triple negativity |
Halogens have a high electron affinity (maximum at
Cl) and very high ionization energy (maximum at F) and maximum
possible electronegativity in each period. Fluorine is the most
electronegative of all chemical elements.
The presence of one unpaired electron in halogen atoms determines
represents the union of atoms in simple substances into diatomic molecules Г2.
For simple substances, halogens, the most characteristic oxidizing agents are
properties, which are strongest in F2 and weaken when moving to I2.
Halogens are characterized by the greatest reactivity of all non-metallic elements. Fluorine, even among halogens, stands out
has extremely high activity.
The element of the second period, fluorine, differs most strongly from the other
other elements of the subgroup. This is a general pattern for all non-metals.
Executor: |
Event No. |
||||||||||||||||
Fluorine, as the most electronegative element, does not show sex
resident oxidation states. In any connection, including with ki-
oxygen, fluorine is in the oxidation state (-1).
All other halogens exhibit positive oxidation degrees
leniya up to a maximum of +7.
The most characteristic oxidation states of halogens:
F: -1, 0;
Cl, Br, I: -1, 0, +1, +3, +5, +7.
Cl has known oxides in which it is found in oxidation states: +4 and +6.
The most important halogen compounds, in positive states,
Penalties of oxidation are oxygen-containing acids and their salts.
All halogen compounds in positive oxidation states are
are strong oxidizing agents.
terrible degree of oxidation. Disproportionation is promoted by an alkaline environment.
Practical application of simple substances and oxygen compounds
The reduction of halogens is mainly due to their oxidizing effect.
The simplest substances, Cl2, find the widest practical application.
and F2. The largest amount of chlorine and fluorine is consumed in industrial
organic synthesis: in the production of plastics, refrigerants, solvents,
pesticides, drugs. Significant amounts of chlorine and iodine are used to obtain metals and for their refining. Chlorine is also used
for bleaching cellulose, for disinfecting drinking water and in production
water of bleach and hydrochloric acid. Salts of oxoacids are used in the production of explosives.
Executor: |
Event No. |
||||||||||||||||

Acids—hydrochloric and molten acids—are widely used in practice.
Fluorine and chlorine are among the twenty most common elements
there, there is significantly less bromine and iodine in nature. All halogens occur in nature in their oxidation state(-1). Only iodine occurs in the form of the salt KIO3,
which is included as an impurity in Chilean saltpeter (KNO3).
Astatine is an artificially produced radioactive element (it does not exist in nature). The instability of At is reflected in the name, which comes from the Greek. "astatos" - "unstable". Astatine is a convenient emitter for radiotherapy of cancer tumors.
Simple substances
Simple substances of halogens are formed by diatomic molecules G2.
In simple substances, during the transition from F2 to I2 with an increase in the number of electrons
throne layers and an increase in the polarizability of atoms, there is an increase
intermolecular interaction, leading to a change in aggregate co-
standing under standard conditions.
Fluorine (under normal conditions) is a yellow gas, at –181o C it turns into
liquid state.
Chlorine is a yellow-green gas that turns into liquid at –34o C. With the color of ha-
The name Cl is associated with it, it comes from the Greek “chloros” - “yellow-
green". A sharp increase in the boiling point of Cl2 compared to F2,
indicates increased intermolecular interaction.
Bromine is a dark red, very volatile liquid, boils at 58.8o C.
the name of the element is associated with the sharp unpleasant odor of gas and is derived from
"bromos" - "smelly".
Iodine – dark purple crystals, with a faint “metallic”
lumps, which when heated easily sublimate, forming violet vapors;
with rapid cooling |
vapors up to 114o C |
liquid is formed. Temperature |
|||||||||||||||||
Executor: |
Event No. |
||||||||||||||||||
The boiling point of iodine is 183 ° C. Its name comes from the color of iodine vapor -
"iodos" - "purple".
All simple substances have a pungent odor and are poisonous.
Inhalation of their vapors causes irritation of the mucous membranes and respiratory organs, and at high concentrations - suffocation. During the First World War, chlorine was used as a poisonous agent.
Fluorine gas and liquid bromine cause skin burns. Working with ha-
logens, precautions should be taken.
Since simple substances of halogens are formed by non-polar molecules
cools, they dissolve well in non-polar organic solvents:
alcohol, benzene, carbon tetrachloride, etc. Chlorine, bromine and iodine are sparingly soluble in water; their aqueous solutions are called chlorine, bromine and iodine water. Br2 dissolves better than others, bromine concentration in sat.
The solution reaches 0.2 mol/l, and chlorine – 0.1 mol/l.
Fluoride decomposes water:
2F2 + 2H2 O = O2 + 4HF
Halogens exhibit high oxidative activity and transition
into halide anions.
Г2 + 2e– 2Г–
Fluorine has especially high oxidative activity. Fluorine oxidizes noble metals (Au, Pt).
Pt + 3F2 = PtF6
It even interacts with some inert gases (krypton,
xenon and radon), for example,
Xe + 2F2 = XeF4
Many very stable compounds burn in an F2 atmosphere, e.g.
water, quartz (SiO2).
SiO2 + 2F2 = SiF4 + O2
Executor: |
Event No. |
||||||||||||||||

In reactions with fluorine, even such strong oxidizing agents as nitrogen and sulfur
nic acid, act as reducing agents, while fluorine oxidizes the input
containing O(–2) in their composition.
2HNO3 + 4F2 = 2NF3 + 2HF + 3O2 H2 SO4 + 4F2 = SF6 + 2HF + 2O2
The high reactivity of F2 creates difficulties with the choice of con-
structural materials for working with it. Usually for these purposes we use
There are nickel and copper, which, when oxidized, form dense protective films of fluorides on their surface. The name F is due to its aggressive action.
I eat, it comes from the Greek. “fluoros” – “destructive”.
In the series F2, Cl2, Br2, I2, the oxidizing ability weakens due to an increase
increasing the size of atoms and decreasing electronegativity.
In aqueous solutions, the oxidative and reductive properties of matter
Substances are usually characterized using electrode potentials. The table shows standard electrode potentials (Eo, V) for reduction half-reactions
formation of halogens. For comparison, the Eo value for ki-
carbon is the most common oxidizing agent.
Standard electrode potentials for simple halogen substances
Eo, B, for reaction |
|||||||||||||
O2 + 4e– + 4H+ 2H2 O |
|||||||||||||
Eo, V |
|||||||||||||
for electrode |
|||||||||||||
2Г– +2е – = Г2 |
|||||||||||||
Reduced oxidative activity
As can be seen from the table, F2 is a much stronger oxidizing agent,
than O2, therefore F2 does not exist in aqueous solutions , it oxidizes water,
recovering to F–. Judging by the Eо value, the oxidizing ability of Cl2
Executor: |
Event No. |
||||||||||||||||
also higher than that of O2. Indeed, during long-term storage of chlorine water, it decomposes with the release of oxygen and the formation of HCl. But the reaction is slow (the Cl2 molecule is noticeably stronger than the F2 molecule and
activation energy for reactions with chlorine is higher), dispro-
portioning:
Cl2 + H2 O HCl + HOCl
In water it does not reach the end (K = 3.9 . 10–4), therefore Cl2 exists in aqueous solutions. Br2 and I2 are characterized by even greater stability in water.
Disproportionation is a very characteristic oxidative
reduction reaction for halogens. Disproportionation of the amplification
pours in an alkaline environment.
Disproportionation of Cl2 in alkali leads to the formation of anions
Cl– and ClO–. The disproportionation constant is 7.5. 1015.
Cl2 + 2NaOH = NaCl + NaClO + H2O
When iodine is disproportioned in alkali, I– and IO3– are formed. Ana-
Logically, Br2 disproportionates iodine. Product change is disproportionate
nation is due to the fact that the anions GO– and GO2– in Br and I are unstable.
The chlorine disproportionation reaction is used in industrial
ability to obtain a strong and fast-acting hypochlorite oxidizer,
bleaching lime, bertholet salt.
3Cl2 + 6 KOH = 5KCl + KClO3 + 3H2 O
Executor: |
Event No. |
||||||||||||||||
Interaction of halogens with metals
Halogens react vigorously with many metals, for example:
Mg + Cl2 = MgCl2 Ti + 2I2 TiI4
Na + halides, in which the metal has a low oxidation state (+1, +2),
- These are salt-like compounds with predominantly ionic bonds. How to
lo, ionic halides are solids with a high melting point
Metal halides in which the metal has a high degree of oxidation
tions are compounds with predominantly covalent bonds.
Many of them are gases, liquids or fusible solids under normal conditions. For example, WF6 is a gas, MoF6 is a liquid,
TiCl4 is liquid.
Interaction of halogens with non-metals
Halogens interact directly with many nonmetals:
hydrogen, phosphorus, sulfur, etc. For example:
H2 + Cl2 = 2HCl 2P + 3Br2 = 2PBr3 S + 3F2 = SF6
The bonding in nonmetal halides is predominantly covalent.
Typically these compounds have low melting and boiling points.
When passing from fluorine to iodine, the covalent nature of the halides increases.
The covalent halides of typical nonmetals are acidic compounds; when interacting with water, they hydrolyze to form acids. For example:
PBr3 + 3H2 O = 3HBr + H3 PO3
PI3 + 3H2 O = 3HI + H3 PO3
PCl5 + 4H2 O = 5HCl + H3 PO4
Executor: |
Event No. |
||||||||||||||||

The first two reactions are used to produce bromine and hydrogen iodide.
noic acid.
Interhalides. Halogens, combining with each other, form interg-
leads. In these compounds, the lighter and more electronegative halogen is in the (–1) oxidation state, and the heavier one is in the positive state.
oxidation penalties.
Due to the direct interaction of halogens upon heating, the following are obtained: ClF, BrF, BrCl, ICl. There are also more complex interhalides:
ClF3, BrF3, BrF5, IF5, IF7, ICl3.
All interhalides under normal conditions are liquid substances with low boiling points. Interhalides have a high oxidative activity
activity. For example, such chemically stable substances as SiO2, Al2 O3, MgO, etc. burn in ClF3 vapors.
2Al2 O3 + 4ClF3 = 4 AlF3 + 3O2 + 2Cl2
Fluoride ClF 3 is an aggressive fluorinating reagent that acts quickly
yard F2. It is used in organic syntheses and to obtain protective films on the surface of nickel equipment for working with fluorine.
In water, interhalides hydrolyze to form acids. For example,
ClF5 + 3H2 O = HClO3 + 5HF
Halogens in nature. Obtaining simple substances
In industry, halogens are obtained from their natural compounds. All
processes for obtaining free halogens are based on the oxidation of halogen
Nid ions.
2Г – Г2 + 2e–
A significant amount of halogens is found in natural waters in the form of anions: Cl–, F–, Br–, I–. Seawater can contain up to 2.5% NaCl.
Bromine and iodine are obtained from oil well water and sea water.
Executor: |
Event No. |
||||||||||||||||
A subgroup of halogens consists of the elements fluorine, chlorine, bromine and iodine.
The electronic configurations of the outer valence layer of halogens are those of fluorine, chlorine, bromine and iodine, respectively). Such electronic configurations determine the typical oxidizing properties of halogens - all halogens have the ability to gain electrons, although when moving to iodine, the oxidizing ability of halogens is weakened.
Under ordinary conditions, halogens exist in the form of simple substances consisting of diatomic molecules of the type with covalent bonds. The physical properties of halogens differ significantly: for example, under normal conditions, fluorine is a gas that is difficult to liquefy, chlorine is also a gas, but liquefies easily, bromine is a liquid, iodine is a solid.
Chemical properties of halogens.
Unlike all other halogens, fluorine in all its compounds exhibits only one oxidation state, 1-, and does not exhibit variable valency. For other halogens, the most characteristic oxidation state is also 1-, however, due to the presence of free -orbitals at the outer level, they can also exhibit other odd oxidation states from to due to partial or complete pairing of valence electrons.
Fluorine has the greatest activity. Most metals, even at room temperature, ignite in its atmosphere, releasing a large amount of heat, for example:
Without heating, fluorine also reacts with many non-metals (hydrogen - see above), while also releasing a large amount of heat:
When heated, fluorine oxidizes all other halogens according to the following scheme:
where , and in the compounds the oxidation states of chlorine, bromine and iodine are equal.
Finally, when irradiated, fluorine reacts even with inert gases:
The interaction of fluorine with complex substances also occurs very vigorously. So, it oxidizes water, and the reaction is explosive:
Free chlorine is also very reactive, although its activity is less than that of fluorine. It reacts directly with all simple substances except oxygen, nitrogen and noble gases, for example:

For these reactions, as for all others, the conditions for their occurrence are very important. Thus, at room temperature, chlorine does not react with hydrogen; when heated, this reaction occurs, but turns out to be highly reversible, and with powerful irradiation it proceeds irreversibly (with an explosion) through a chain mechanism.
Chlorine reacts with many complex substances, for example, substitution and addition with hydrocarbons:

Chlorine is capable of upon heating, displace bromine or iodine from their compounds with hydrogen or metals:

and also reacts reversibly with water:
Chlorine, dissolving in water and partially reacting with it, as shown above, forms an equilibrium mixture of substances called chlorine water.
Note also that chlorine on the left side of the last equation has an oxidation state of 0. As a result of the reaction, the oxidation state of some chlorine atoms became 1- (in), for others (in hypochlorous acid). This reaction is an example of a self-oxidation-self-reduction reaction, or disproportionation.
Let us recall that chlorine can react (disproportionate) with alkalis in the same way (see the section “Bases” in § 8).
The chemical activity of bromine is less than fluorine and chlorine, but is still quite high due to the fact that bromine is usually used in a liquid state and therefore its initial concentrations, other things being equal, are greater than those of chlorine. Being a “softer” reagent, bromine is widely used in organic chemistry.
Note that bromine, like chlorine, dissolves in water and, partially reacting with it, forms the so-called “bromine water”, while iodine is practically insoluble in water and is not capable of oxidizing it even when heated; for this reason there is no “iodine water”.
Production of halogens.
The most common technological method for producing fluorine and chlorine is the electrolysis of molten salts (see § 7). Bromine and iodine in industry are usually obtained chemically.
In the laboratory, chlorine is produced by the action of various oxidizing agents on hydrochloric acid, for example:
Oxidation is carried out even more efficiently with potassium permanganate - see the section “Acids” in § 8.
Hydrogen halides and hydrohalic acids.
All hydrogen halides are gaseous under normal conditions. The chemical bond carried out in their molecules is polar covalent, and the polarity of the bond decreases in the series. The bond strength also decreases in this series. Due to their polarity, all hydrogen halides, unlike halogens, are highly soluble in water. So, at room temperature in 1 volume of water you can dissolve about 400 volumes of volumes and about 400 volumes of
When hydrogen halides are dissolved in water, they dissociate into ions, and solutions of the corresponding hydrohalide acids are formed. Moreover, upon dissolution, HCI dissociates almost completely, so the resulting acids are considered strong. In contrast, hydrofluoric acid is weak. This is explained by the association of HF molecules due to the occurrence of hydrogen bonds between them. Thus, the strength of acids decreases from HI to HF.
Since negative ions of hydrohalic acids can only exhibit reducing properties, when these acids interact with metals, the oxidation of the latter can occur only due to ions. Therefore, acids react only with metals that are in the voltage series to the left of hydrogen.
All metal halides, with the exception of Ag and Pb salts, are highly soluble in water. The low solubility of silver halides allows the use of an exchange reaction like
as qualitative for the detection of the corresponding ions. As a result of the reaction, AgCl precipitates as a white precipitate, AgBr - yellowish-white, Agl - bright yellow.
Unlike other hydrohalic acids, hydrofluoric acid reacts with silicon (IV) oxide:
Since silicon oxide is part of glass, hydrofluoric acid corrodes glass, and therefore in laboratories it is stored in containers made of polyethylene or Teflon.
All halogens, except fluorine, can form compounds in which they have a positive oxidation state. The most important of these compounds are the oxygen-containing acids of the halogen type and their corresponding salts and anhydrides.
The hydrogen atom has the electronic formula of the outer (and only) electron level 1 s 1 . On the one hand, in terms of the presence of one electron on the outer electronic level, the hydrogen atom is similar to alkali metal atoms. However, just like halogens, it only needs one electron to fill the outer electronic level, since the first electronic level can contain no more than 2 electrons. It turns out that hydrogen can be placed simultaneously in both the first and the penultimate (seventh) group of the periodic table, which is sometimes done in various versions of the periodic system:
From the point of view of the properties of hydrogen as a simple substance, it still has more in common with halogens. Hydrogen, like halogens, is a non-metal and forms diatomic molecules (H 2) like them.
Under normal conditions, hydrogen is a gaseous, low-active substance. The low activity of hydrogen is explained by the high strength of the bonds between the hydrogen atoms in the molecule, the breaking of which requires either strong heating, or the use of catalysts, or both.
Interaction of hydrogen with simple substances
with metals
Of the metals, hydrogen reacts only with alkali and alkaline earth metals! Alkali metals include metals of the main subgroup of group I (Li, Na, K, Rb, Cs, Fr), and alkaline earth metals include metals of the main subgroup of group II, except beryllium and magnesium (Ca, Sr, Ba, Ra)
When interacting with active metals, hydrogen exhibits oxidizing properties, i.e. lowers its oxidation state. In this case, hydrides of alkali and alkaline earth metals are formed, which have an ionic structure. The reaction occurs when heated:
It should be noted that interaction with active metals is the only case when molecular hydrogen H2 is an oxidizing agent.
with non-metals
Of the non-metals, hydrogen reacts only with carbon, nitrogen, oxygen, sulfur, selenium and halogens!
Carbon should be understood as graphite or amorphous carbon, since diamond is an extremely inert allotropic modification of carbon.
When interacting with non-metals, hydrogen can only perform the function of a reducing agent, that is, only increase its oxidation state:
Interaction of hydrogen with complex substances
with metal oxides
Hydrogen does not react with metal oxides that are in the activity series of metals up to aluminum (inclusive), however, it is capable of reducing many metal oxides to the right of aluminum when heated:
with non-metal oxides
Of the non-metal oxides, hydrogen reacts when heated with the oxides of nitrogen, halogens and carbon. Of all the interactions of hydrogen with non-metal oxides, especially noteworthy is its reaction with carbon monoxide CO.
The mixture of CO and H2 even has its own name - “synthesis gas”, since, depending on the conditions, such popular industrial products as methanol, formaldehyde and even synthetic hydrocarbons can be obtained from it:
with acids
Hydrogen does not react with inorganic acids!
Of organic acids, hydrogen reacts only with unsaturated acids, as well as with acids containing functional groups capable of reduction with hydrogen, in particular aldehyde, keto or nitro groups.
with salts
In the case of aqueous solutions of salts, their interaction with hydrogen does not occur. However, when hydrogen is passed over solid salts of some metals of medium and low activity, their partial or complete reduction is possible, for example:
Chemical properties of halogens
Halogens are the chemical elements of group VIIA (F, Cl, Br, I, At), as well as the simple substances they form. Here and further in the text, unless otherwise stated, halogens will be understood as simple substances.
All halogens have a molecular structure, which determines the low melting and boiling points of these substances. Halogen molecules are diatomic, i.e. their formula can be written in general form as Hal 2.
It should be noted such a specific physical property of iodine as its ability to sublimation or, in other words, sublimation. Sublimation, is a phenomenon in which a substance in a solid state does not melt when heated, but, bypassing the liquid phase, immediately passes into the gaseous state.
The electronic structure of the external energy level of an atom of any halogen has the form ns 2 np 5, where n is the number of the periodic table period in which the halogen is located. As you can see, the halogen atoms only need one electron to reach the eight-electron outer shell. From this it is logical to assume the predominantly oxidizing properties of free halogens, which is confirmed in practice. As is known, the electronegativity of nonmetals decreases when moving down a subgroup, and therefore the activity of halogens decreases in the series:
F 2 > Cl 2 > Br 2 > I 2
Interaction of halogens with simple substances
All halogens are highly reactive substances and react with most simple substances. However, it should be noted that fluorine, due to its extremely high reactivity, can react even with those simple substances with which other halogens cannot react. Such simple substances include oxygen, carbon (diamond), nitrogen, platinum, gold and some noble gases (xenon and krypton). Those. actually, fluorine does not react only with some noble gases.
The remaining halogens, i.e. chlorine, bromine and iodine are also active substances, but less active than fluorine. They react with almost all simple substances except oxygen, nitrogen, carbon in the form of diamond, platinum, gold and noble gases.
Interaction of halogens with non-metals
hydrogen
When all halogens interact with hydrogen, they form hydrogen halides with the general formula HHal. In this case, the reaction of fluorine with hydrogen begins spontaneously even in the dark and proceeds with an explosion in accordance with the equation:
The reaction of chlorine with hydrogen can be initiated by intense ultraviolet irradiation or heat. Also proceeds with explosion:
Bromine and iodine react with hydrogen only when heated, and at the same time, the reaction with iodine is reversible:
phosphorus
The interaction of fluorine with phosphorus leads to the oxidation of phosphorus to the highest oxidation state (+5). In this case, phosphorus pentafluoride is formed:
When chlorine and bromine interact with phosphorus, it is possible to obtain phosphorus halides both in the oxidation state + 3 and in the oxidation state +5, which depends on the proportions of the reacting substances:
Moreover, in the case of white phosphorus in an atmosphere of fluorine, chlorine or liquid bromine, the reaction begins spontaneously.
The interaction of phosphorus with iodine can lead to the formation of only phosphorus triodide due to its significantly lower oxidizing ability than that of other halogens:
gray
Fluorine oxidizes sulfur to the highest oxidation state +6, forming sulfur hexafluoride:
Chlorine and bromine react with sulfur, forming compounds containing sulfur in the oxidation states +1 and +2, which are extremely unusual for it. These interactions are very specific, and to pass the Unified State Exam in chemistry, the ability to write equations for these interactions is not necessary. Therefore, the following three equations are given rather for reference:
Interaction of halogens with metals
As mentioned above, fluorine is capable of reacting with all metals, even such inactive ones as platinum and gold:
The remaining halogens react with all metals except platinum and gold:
Reactions of halogens with complex substances
Substitution reactions with halogens
More active halogens, i.e. the chemical elements of which are located higher in the periodic table are capable of displacing less active halogens from the hydrohalic acids and metal halides they form:
Similarly, bromine and iodine displace sulfur from solutions of sulfides and or hydrogen sulfide:
Chlorine is a stronger oxidizing agent and oxidizes hydrogen sulfide in its aqueous solution not to sulfur, but to sulfuric acid:
Reaction of halogens with water
Water burns in fluorine with a blue flame in accordance with the reaction equation:
Bromine and chlorine react differently with water than fluorine. If fluorine acted as an oxidizing agent, then chlorine and bromine are disproportionate in water, forming a mixture of acids. In this case, the reactions are reversible:
The interaction of iodine with water occurs to such an insignificant degree that it can be neglected and it can be assumed that the reaction does not occur at all.
Interaction of halogens with alkali solutions
Fluorine, when interacting with an aqueous alkali solution, again acts as an oxidizing agent:
The ability to write this equation is not required to pass the Unified State Exam. It is enough to know the fact about the possibility of such an interaction and the oxidative role of fluorine in this reaction.
Unlike fluorine, other halogens in alkali solutions are disproportionate, that is, they simultaneously increase and decrease their oxidation state. Moreover, in the case of chlorine and bromine, depending on the temperature, flow in two different directions is possible. In particular, in the cold the reactions proceed as follows:
and when heated:
Iodine reacts with alkalis exclusively according to the second option, i.e. with the formation of iodate, because hypoiodite is not stable not only when heated, but also at ordinary temperatures and even in the cold.
The halogens fluorine F, chlorine C1, bromine Br, iodine I are elements of the VILA group. Electronic configuration of the valence shell of halogen atoms in the ground state ns 2 np 5 . The presence of five electrons in the outer p orbital, including one unpaired one, is the reason for the high electron affinity of halogens. The addition of an electron leads to the formation of halide anions (F-, Cl-, Br-, I-) with a stable 8-electron shell of the nearest noble gas. Halogens are distinct non-metals.
The most electronegative element, fluorine, has only one oxidation state in compounds - 1, since it is always an electron acceptor. Other halogens in compounds can have oxidation states ranging from -1 to +7. The positive oxidation states of halogens are caused by the transition of their valence electrons to free d-orbitals of the outer level (Section 2.1.3) upon the formation of bonds with more electronegative elements.
The halogen molecules are diatomic: F 2, C1 2, Br 2, I 2. Under standard conditions, fluorine and chlorine are gases, bromine is a volatile liquid (Tbp = 59 °C), and iodine is a solid, but it easily sublimes (transforms into a gaseous state, bypassing the liquid state).
Redox properties. Halogens are strong oxidizing agents, reacting with almost all metals and many non-metals:
Fluorine exhibits especially high chemical activity, which, when heated, reacts even with the noble gases xenon, krypton and radon:
![]()
The chemical activity of halogens decreases from fluorine to iodine, since with increasing atomic radius the ability of halogens to attach electrons decreases:

The more active halogen always displaces the less active one from its compounds with metals. Thus, fluorine displaces all other halogens from their halides, and bromine displaces only iodine from iodides:
The different oxidative properties of halogens are also manifested in their effect on the body. Gaseous chlorine and fluorine, due to their very strong oxidizing properties, are powerful toxic substances that cause severe damage to the lungs and mucous membranes of the eyes, nose and larynx. Iodine is a milder oxidizing agent that exhibits antiseptic properties, so it is widely used in medicine.
Differences in the redox properties of halogens also appear when they interact with water. Fluorine oxidizes water, with the reducing agent being the oxygen atom of the water molecule:
The interaction of other halogens with water is accompanied by redox dismutation of their atoms. Thus, when chlorine reacts with water, one of the atoms of the chlorine molecule, gaining an electron from another atom, is reduced, and the other chlorine atom, giving up an electron, is oxidized. This creates chlorine water, containing hydrogen chloride (hydrochloric acid) and hypochlorous (hypochlorous) acid:
The reaction is reversible, and its equilibrium is strongly shifted to the left. Hypochlorous acid is unstable and easily decomposes, especially in light, with the formation of a very strong oxidizing agent - atomic oxygen:
Thus, chlorine water contains in different concentrations three oxidizing agents with different oxidizing abilities: molecular chlorine, hypochlorous acid and atomic oxygen, the sum of which is often called "active chlorine".
The resulting atomic oxygen bleaches dyes and kills microbes, which explains the bleaching and bactericidal effect of chlorine water.
Hypochlorous acid is a stronger oxidizing agent than chlorine gas. It reacts with organic compounds RH both as an oxidizing agent and as a chlorinating reagent:
Therefore, when drinking water containing organic substances as impurities is chlorinated, they can turn into more toxic organochlorine compounds RC1. This should definitely be taken into account when developing water purification methods and their application.
When alkali is added to chlorine water, the equilibrium shifts to the right due to the neutralization of hypochlorous and hydrochloric acids:
The resulting solution of a mixture of salts, called Javel water, used as a bleaching and disinfectant. These properties are due to the fact that potassium hypochlorite under the influence of CO2 + H 2 0 and as a result of hydrolysis is converted into unstable hypochlorous acid, forming atomic oxygen. As a result, Javel water destroys dyes and kills microbes.
When gaseous chlorine acts on wet slaked lime Ca(OH) 2, a mixture of salts CaCl 2 and Ca(0C1) 2 is obtained, called bleach:
Chloride of lime can be considered as a mixed calcium salt of hydrochloric and hypochlorous acids CaCl(OCl). In humid air, bleach, interacting with water and carbon dioxide, gradually releases hypochlorous acid, which provides its bleaching, disinfectant and degassing properties:
When bleach is exposed to hydrochloric acid, free chlorine is released:
When heated, hypochlorous acid decomposes as a result of redox disproportionation to form hydrochloric and perchloric acids:
![]()
When chlorine is passed through a hot alkali solution, such as KOH, potassium chloride and potassium chlorate KClO 3 (Berthollet salt) are formed:
The oxidizing ability of anions of oxygen-containing chlorine acids in aqueous solutions in the series СlO - - СlO4(-) decreases despite the increase in the oxidation degree of chlorine in them:
![]()
This is explained by an increase in the stability of anions in this series due to increased delocalization of their negative charge. At the same time, LiC10 4 and KClO 4 perchlorates in the dry state at high temperatures are strong oxidizing agents and are used for the mineralization of various biomaterials when determining the inorganic components they contain.
Halogen anions (except F-) are capable of donating electrons, so they are reducing agents. As their radius increases, the reducing ability of halide anions increases from the chloride anion to the iodide anion:

Thus, hydroiodic acid is oxidized by atmospheric oxygen already at normal temperature:
![]()
Hydrochloric acid is not oxidized by oxygen, and therefore the chloride anion is stable under body conditions, which is very important from the standpoint of physiology and medicine.
Acid-base properties. Hydrogen halides HF, HC1, HBr, HI, due to the polarity of their molecules, are highly soluble in water. In this case, hydration of the molecules occurs, leading to their dissociation with the formation of hydrated protons and halide anions. The strength of acids in the series HF, HC1, HBr, HI increases due to an increase in the radius and polarizability of anions from F- to I-.
Hydrochloric acid, as a component of gastric juice, plays an important role in the digestion process. Mainly due to hydrochloric acid, the mass fraction of which in gastric juice is 0.3%, its pH is maintained in the range from 1 to 3. Hydrochloric acid promotes the transition of the pepsin enzyme to its active form, which ensures the digestion of proteins due to the hydrolytic cleavage of peptide bonds with formation of various amino acids:
The determination of the content of hydrochloric acid and other acids in gastric juice was discussed in section. 8.3.3.
In the series of oxygen-containing acids of chlorine, as its oxidation state increases, the strength of the acids increases.

This is due to an increase in the polarity of the O-H bond due to a shift in its electron density towards the chlorine atom, as well as due to an increase in the stability of the anions.
Complexing properties. Halogen anions tend to form complexes as ligands. The stability of halide complexes usually decreases in the order F- > Cl- > Br- > > I-. It is the process of complex formation that explains the toxic effect of fluoride anions, which, by forming fluoride complexes with metal cations included in the active centers of enzymes, suppress their activity.
The iodine molecule exhibits interesting complex-forming properties. Thus, the solubility of molecular iodine in water increases sharply in the presence of potassium iodide, which is associated with the formation of a complex anion
The low stability of this complex ion ensures the presence of molecular iodine in solution. Therefore, in medicine, an aqueous solution of iodine with the addition of KI is used as a bactericidal agent. In addition, molecular iodine forms inclusion complexes with starch (Section 22.3) and polyvinyl alcohol (blue iodine). In these complexes, iodine molecules or their associates with iodide anions fill the channels formed by the helical structure of the corresponding polyhydroxy polymers. Inclusion complexes are not very stable and are capable of gradually releasing molecular iodine. Therefore, a drug such as blue iodine is an effective, but mild, long-acting bactericidal agent.
Biological role and use of halogens and their compounds in medicine. Halogens in the form of various compounds are part of living tissues. In the body, all halogens have an oxidation state of 1. At the same time, chlorine and bromine exist in the form of hydrated Cl- and Br- anions, and fluorine and iodine are part of water-insoluble biosubstrates:
Fluorine compounds are components of bone tissue, nails and teeth. The biological effect of fluoride is primarily associated with the problem of dental diseases. The fluoride anion, replacing the hydroxide ion in hydroxyapatite, forms a layer of protective enamel from solid fluorapatite:
Fluoridation of drinking water to a fluoride ion concentration of 1 mg/l and the addition of sodium fluoride to toothpaste significantly reduce dental caries in the population. At the same time, when the concentration of fluoride anion in drinking water is above 1.2 mg/l, the fragility of bones and tooth enamel increases and general exhaustion of the body appears, called fluorosis.
Chloride anions provide ionic flows through cell membranes, participate in maintaining osmotic homeostasis, and create a favorable environment for the action and activation of protolytic enzymes of gastric juice.
Bromide anions in the human body are localized mainly in the pituitary gland and other endocrine glands. The presence of a dynamic relationship between the content of bromide and chloride anions in the body has been established. Thus, the increased content of bromide anions in the blood promotes the rapid release of chloride anions by the kidneys. Bromides are localized mainly in the intercellular fluid. They enhance inhibitory processes in the neurons of the cerebral cortex, and therefore potassium, sodium and bromocamphor bromides are used in pharmacology.
Iodine and its compounds affect the synthesis of proteins, fats and hormones. More than half of the amount of iodine is in the thyroid gland in a bound state in the form of thyroid hormones. With insufficient intake of iodine in the body, endemic goiter develops. In order to prevent this disease, NaI or KI is added to table salt (1-2 g per 1 kg of NaCl). Thus, all halogens are necessary for the normal functioning of living organisms.
Chapter 13
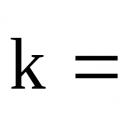 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe