Atomic weight of chromium. Chromium - general characteristics of the element, chemical properties of chromium and its compounds
Chromium
Element No. 24. One of the hardest metals. Has high chemical resistance. One of the most important metals used in the production of alloy steels. Most chromium compounds are brightly colored and come in a variety of colors. For this feature, the element was named chromium, which means “paint” in Greek.
How was he found?
A mineral containing chromium was discovered near Yekaterinburg in 1766 by I.G. Lehmann called it “Siberian red lead”. Now this mineral is called crocoite. Its composition is also known - PbCrO 4. And at one time, “Siberian red lead” caused a lot of disagreement among scientists. For thirty years they argued about its composition, until, finally, in 1797, the French chemist Louis Nicolas Vauquelin isolated a metal from it, which (also, by the way, after some controversy) was called chromium.
Vauquelin treated crocoite with potash K 2 CO 3: lead chromate turned into potassium chromate. Potassium chromate was then converted into chromium oxide and water using hydrochloric acid (chromic acid exists only in dilute solutions). By heating green chromium oxide powder in a graphite crucible with coal, Vauquelin obtained a new refractory metal.
The Paris Academy of Sciences witnessed the discovery in its entirety. But, most likely, Vauquelin isolated not elemental chromium, but its carbides. This is evidenced by the needle-shaped shape of the light gray crystals obtained by Vauquelin.
The name “chrome” was suggested by Vauquelin’s friends, but he did not like it - the metal did not have a special color. However, friends managed to persuade the chemist, citing the fact that brightly colored chromium compounds can be used to obtain good paints. (By the way, it was in the works of Vauquelin that the emerald color of some natural beryllium and aluminum silicates was first explained; they, as Vauquelin found out, were colored by impurities of chromium compounds.) And so this name was adopted for the new element.
By the way, the syllable “chrome”, precisely in the sense of “colored”, is included in many scientific, technical and even musical terms. Isopanchrome, panchrome and orthochrome photographic films are widely known. The word "chromosome" translated from Greek means "body that is colored." There is a “chromatic” scale (in music) and there is a “chromatic” harmonic.
Where is he located
IN earth's crust There is quite a lot of chromium - 0.02%. The main mineral from which the industry obtains chromium is chrome spinel of variable composition with general formula(Mg, Fe) O · (Cr, Al, Fe) 2 O 3 . Chrome ore is called chromite or chromium iron ore (because it almost always contains iron). There are deposits of chrome ores in many places. Our country has huge reserves of chromites. One of the largest deposits is located in Kazakhstan, in the Aktobe region; it was discovered in 1936. There are significant reserves of chrome ores in the Urals.
Chromites are mostly used for smelting ferrochrome. It is one of the most important ferroalloys, absolutely necessary for the mass production of alloy steels.
Ferroalloys are alloys of iron with other elements used mainly for alloying and deoxidizing steel. Ferrochrome contains at least 60% Cr.
Tsarist Russia produced almost no ferroalloys. Several blast furnaces at southern factories smelted low-percentage (alloying metal) ferrosilicon and ferromanganese. Moreover, on the Satka River, which flows in the Southern Urals, in 1910 a tiny factory was built that smelted tiny amounts of ferromanganese and ferrochrome.
In the first years of development, the young Soviet country had to import ferroalloys from abroad. Such dependence on capitalist countries was unacceptable. Already in 1927...1928. The construction of Soviet ferroalloy plants began. At the end of 1930, the first large ferroalloy furnace was built in Chelyabinsk, and in 1931 the Chelyabinsk plant, the first-born of the ferroalloy industry of the USSR, came into operation. In 1933, two more factories were launched - in Zaporozhye and Zestafoni. This made it possible to stop the import of ferroalloys. In just a few years, the Soviet Union organized the production of many types of special steels - ball bearing, heat-resistant, stainless, automotive, high-speed... All these steels contain chromium.
At the 17th Party Congress, People's Commissar of Heavy Industry Sergo Ordzhonikidze said: “...if we did not have high-quality steels, we would not have an automobile and tractor industry. The cost of the high-quality steel we currently use is estimated at over 400 million rubles. If it were necessary to import, it would be 400 million rubles. every year, damn it, you would end up in bondage to the capitalists...”
The plant on the basis of the Aktobe field was built later, during the Great Patriotic War. He produced the first ferrochrome smelting on January 20, 1943. The workers of the city of Aktyubinsk took part in the construction of the plant. The construction was declared public. The ferrochrome of the new plant was used to produce metal for tanks and guns, for the needs of the front.
Years have passed. Now the Aktobe Ferroalloy Plant is the largest enterprise producing ferrochrome of all grades. The plant has produced highly qualified national metallurgical personnel. From year to year, the plant and chromite mines are increasing their capacity, providing our ferrous metallurgy with high-quality ferrochrome.
Our country has a unique deposit of naturally alloyed iron ores rich in chromium and nickel. It is located in the Orenburg steppes. The Orsko-Khalilovsky Metallurgical Plant was built and operates on the basis of this deposit. Naturally alloyed cast iron, which has high heat resistance, is smelted in the plant's blast furnaces. Part of it is used in the form of casting, but most of it is sent for processing into nickel steel; chromium burns out when smelting steel from cast iron.
Cuba, Yugoslavia, and many countries in Asia and Africa have large reserves of chromites.
How do you get it?
Chromite is used primarily in three industries: metallurgy, chemistry, and refractories, with metallurgy consuming approximately two-thirds of all chromite.
Steel alloyed with chromium has increased strength and resistance to corrosion in aggressive and oxidizing environments.
Obtaining pure chromium is an expensive and labor-intensive process. Therefore, for alloying steel, ferrochrome is mainly used, which is obtained in electric arc furnaces directly from chromite. The reducing agent is coke. The chromium oxide content in chromite must be at least 48%, and the Cr:Fe ratio must be at least 3:1.
Ferrochrome produced in an electric furnace usually contains up to 80% chromium and 4...7% carbon (the rest is iron).
But to alloy many high-quality steels, you need ferrochrome, which contains little carbon (the reasons for this are discussed below, in the chapter “Chrome in alloys”). Therefore, part of the high-carbon ferrochrome is subjected to special treatment to reduce the carbon content in it to tenths and hundredths of a percent.
Elementary metallic chromium is also obtained from chromite. The production of technically pure chromium (97...99%) is based on the aluminothermy method, discovered back in 1865 by the famous Russian chemist N.N. Beketov. The essence of the method is the reduction of oxides with aluminum; the reaction is accompanied by a significant release of heat.
But first you need to obtain pure chromium oxide Cr 2 O 3. To do this, finely ground chromite is mixed with soda and limestone or iron oxide is added to this mixture. The entire mass is burned, and sodium chromate is formed:
2Cr 2 O 3 + 4Na 2 CO 3 + 3O 2 > 4Na 2 CrO 4 + 4CO 2.
Sodium chromate is then leached from the calcined mass with water; the liquor is filtered, evaporated and treated with acid. The result is sodium bichromate Na 2 Cr 2 O 7 . By reducing it with sulfur or carbon when heated, green chromium oxide is obtained.
Metallic chromium can be obtained by mixing pure chromium oxide with aluminum powder, heating this mixture in a crucible to 500...600°C and igniting it with barium peroxide. Aluminum takes oxygen away from chromium oxide. This reaction Cr 2 O 3 + 2Al > Al 2 O 3 + 2Сr is the basis of the industrial (aluminothermic) method for producing chromium, although, of course, the factory technology is much more complicated. Chromium obtained aluminothermically contains tenths of a percent of aluminum and iron, and hundredths of a percent of silicon, carbon and sulfur.
A silicothermic method is also used to obtain technically pure chromium. In this case, chromium is reduced from oxide by silicon according to the reaction
2Сr 2 О 3 + 3Si > 3SiO 2 + 4Сr.
This reaction occurs in arc furnaces. To bind silica, limestone is added to the charge. The purity of silicothermic chromium is approximately the same as aluminothermic chromium, although, of course, the silicon content in it is slightly higher and the aluminum content is slightly lower. To obtain chromium, they also tried to use other reducing agents - carbon, hydrogen, magnesium. However, these methods are not widely used.
High purity chromium (approximately 99.8%) is obtained electrolytically.
Technically pure and electrolytic chromium is used mainly for the production of complex chromium alloys.
Constants and properties of chromium
The atomic mass of chromium is 51.996. In the periodic table it occupies a place in the sixth group. Its closest neighbors and analogues are molybdenum and tungsten. It is characteristic that chromium's neighbors, like chromium itself, are widely used for alloying steels.
The melting point of chromium depends on its purity. Many researchers have tried to determine it and obtained values from 1513 to 1920°C. Such a large “scatter” is explained primarily by the amount and composition of impurities contained in chromium. It is now believed that chromium melts at a temperature of about 1875°C. Boiling point 2199°C. The density of chromium is less than that of iron; it is equal to 7.19.
In terms of chemical properties, chromium is close to molybdenum and tungsten. Its highest oxide CrO 3 is acidic, it is chromic acid anhydride H 2 CrO 4. The mineral crocoite, with which we began our acquaintance with element No. 24, is a salt of this acid. In addition to chromic acid, dichromic acid H 2 Cr 2 O 7 is known; its salts - bichromates - are widely used in chemistry. The most common chromium oxide, Cr 2 O 3, is amphoteric. In general, under different conditions, chromium can exhibit valencies from 2 to 6. Only compounds of tri- and hexavalent chromium are widely used.
Chrome has all the properties of a metal - it conducts heat well and electricity, has a characteristic metallic luster. The main feature of chromium is its resistance to acids and oxygen.
For those who constantly deal with chromium, another of its features has become the talk of the town: at a temperature of about 37°C, some of the physical properties of this metal change sharply and abruptly. At this temperature there is a clearly expressed maximum internal friction and a minimum elastic modulus. Electrical resistance, coefficient of linear expansion, and thermoelectromotive force change almost as sharply.
Scientists cannot yet explain this anomaly.
There are four known natural isotopes of chromium. Their mass numbers are 50, 52, 53 and 54. The share of the most common isotope 52 Cr is about 84%
Chrome in alloys
It would probably be unnatural if the story about the use of chromium and its compounds began not with steel, but with something else. Chromium is one of the most important alloying elements used in ferrous metallurgy. The addition of chromium to conventional steels (up to 5% Cr) improves their physical properties and makes the metal more susceptible to heat treatment. Spring, spring, tool, stamp and ball bearing steels are alloyed with chromium. In them (except for ball bearing steels) chromium is present along with manganese, molybdenum, nickel, and vanadium. And ball bearing steels contain only chromium (about 1.5%) and carbon (about 1%). The latter forms carbides of exceptional hardness with chromium: Cr 3 C. Cr 7 C 3 and Cr 23 C 6. They give ball bearing steel high wear resistance.
If the chromium content of steel is increased to 10% or more, the steel becomes more resistant to oxidation and corrosion, but this is where a factor that can be called carbon limitation comes into play. The ability of carbon to bind large amounts of chromium leads to the depletion of steel in this element. Therefore, metallurgists are faced with a dilemma: if you want to gain corrosion resistance, reduce the carbon content and lose on wear resistance and hardness.
The most common grade of stainless steel contains 18% chromium and 8% nickel. The carbon content in it is very low - up to 0.1%. Stainless steels resist corrosion and oxidation well and retain strength at high temperatures. The sculptural group of V.I. was made from sheets of such steel. Mukhina “Worker and Collective Farm Woman”, which is installed in Moscow at the Northern entrance to the Exhibition of Achievements National economy. Stainless steels are widely used in the chemical and petroleum industries.
High-chromium steels (containing 25...30% Cr) are particularly resistant to oxidation at high temperatures. They are used for the manufacture of parts for heating furnaces.
Now a few words about chromium-based alloys. These are alloys containing more than 50% chromium. They have very high heat resistance. However, they have a very big drawback that negates all the advantages: these alloys are very sensitive to surface defects: it is enough for a scratch or microcrack to appear, and the product will quickly collapse under load. For most alloys, such deficiencies are eliminated by thermomechanical treatment, but chromium-based alloys cannot be treated in this way. In addition, they are too brittle at room temperature, which also limits their application.
Alloys of chromium and nickel are more valuable (they often contain alloying additives and other elements). The most common alloys of this group - nichromes contain up to 20% chromium (the rest is nickel) and are used for the manufacture of heating elements. Nichromes have high electrical resistance for metals; when current is passed through, they become very hot.
The addition of molybdenum and cobalt to chromium-nickel alloys makes it possible to obtain materials with high heat resistance and the ability to withstand heavy loads at 650...900°C. For example, gas turbine blades are made from these alloys.
Cobalt-chromium alloys containing 25...30% chromium also have heat resistance. Industry also uses chromium as a material for anti-corrosion and decorative coatings.
The main chrome ore, chromite, is also used in the production of refractories. Magnesite-chromite bricks are chemically passive and heat-resistant, they can withstand repeated sudden temperature changes. Therefore, they are used in the designs of open-hearth furnace roofs. The durability of magnesite-chromite vaults is 2...3 times greater than that of dinas vaults.
Dinas is an acidic refractory brick containing at least 93% silica. Fire resistance of dinas is 1680...1730°C. In the 14th volume of the Bolshoi, published in 1952, Soviet Encyclopedia(2nd edition) dinas is called an indispensable material for the arches of open-hearth furnaces. This statement should be considered outdated, although dinas is still widely used as a refractory.
Chemists mainly obtain potassium and sodium bichromates K 2 Cr 2 O 7 and Na 2 Cr 2 O 7 from chromite.
Bpchromates and chrome alum KCr(SO 4); used for tanning leather. This is where the name “chrome” boots comes from. Leather. tanned with chrome compounds, has a beautiful shine, is durable and easy to use.
From lead chromate PbCrO 4. produce various dyes. A solution of sodium dichromate is used to clean and etch the surface of steel wire before galvanizing, and also to brighten brass. Chromite and other chromium compounds are widely used as colorants for ceramic glazes and glass.
Finally, chromic acid is obtained from sodium dichromate, which is used as an electrolyte in the chrome plating of metal parts.
Chromium will continue to remain important in the future as an alloying additive to steel and as a material for metal coatings; Chromium compounds used in the chemical and refractory industries will not lose their value.
The situation is much more complicated with chromium-based alloys. The great fragility and exceptional complexity of machining do not yet allow these alloys to be widely used, although in terms of heat resistance and wear resistance they can compete with any materials. IN last years A new direction has emerged in the production of chromium-containing alloys - alloying them with nitrogen. This gas, usually harmful in metallurgy, forms strong compounds with chromium - nitrides. Nitriding of chromium steels increases their wear resistance and makes it possible to reduce the content of scarce nickel in “stainless steels”. Perhaps this method will also overcome the “unprocessability” of chromium-based alloys? Or will other, as yet unknown methods come to the rescue? One way or another, we must think that in the future these alloys will take their rightful place among the materials needed by technology.
Three or six?
Because chromium resists oxidation in air and acids, it is often applied to the surface of other materials to protect them from corrosion. The application method has long been known - this is electrolytic deposition. However, at first, unexpected difficulties arose during the development of the electrolytic chromium plating process.
It is known that conventional electroplating is applied using electrolytes in which the ion of the element being deposited has a positive charge. This did not work with chrome: the coatings turned out to be porous and peeled off easily.
For almost three quarters of a century, scientists worked on the problem of chrome plating and only in the 20s of our century they found that the electrolyte of a chrome bath should contain not trivalent chromium, but chromic acid, i.e. hexavalent chromium. During industrial chrome plating, salts of sulfuric and hydrofluoric acids are added to the bath; free acid radicals catalyze the process of galvanic deposition of chromium.
Scientists have not yet come to a consensus on the mechanism of deposition of hexavalent chromium on the cathode of a galvanic bath. There is an assumption that hexavalent chromium first transforms into trivalent chromium, and then is reduced to metal. However, most experts agree that chromium at the cathode is reduced immediately from the hexavalent state. Some scientists believe that atomic hydrogen is involved in this process, while others believe that hexavalent chromium simply gains six electrons.
Decorative and solid
There are two types of chrome coatings: decorative and hard. More often you come across decorative ones: on watches, door handles and other objects. Here, a layer of chromium is applied to an underlayer of another metal, most often nickel or copper. The steel is protected from corrosion by this sublayer, and a thin (0.0002...0.0005 mm) layer of chrome gives the product a formal appearance.
Hard surfaces are built differently. Chromium is applied to steel in a much thicker layer (up to 0.1 mm), but without sublayers. Such coatings increase the hardness and wear resistance of steel, and also reduce the coefficient of friction.
Chrome plating without electrolyte
There is another method of applying chrome coatings - diffusion. This process does not take place in galvanic baths, but in furnaces.
The steel piece is placed in chromium powder and heated in a reducing atmosphere. In 4 hours at a temperature of 1300°C, a chromium-enriched layer 0.08 mm thick is formed on the surface of the part. The hardness and corrosion resistance of this layer is much greater than the hardness of the steel in the mass of the part. But this seemingly simple method had to be improved several times. Chromium carbides formed on the surface of the steel, which prevented the diffusion of chromium into the steel. In addition, chromium powder sinteres at temperatures of about a thousand degrees. To prevent this from happening, neutral refractory powder is added to it. Attempts to replace chromium powder with a mixture of chromium oxide and coal did not produce positive results.
A more viable proposal was to use its volatile halide salts, for example CrCl 2 , as a chromium carrier. Hot gas washes the chrome-plated product, and the reaction occurs:
СrСl 2 + Fe - FeСl 2 + Сr.
The use of volatile halide salts made it possible to reduce the chromium plating temperature.
Chromium chloride (or iodide) is usually obtained in the chromium plating plant itself, by passing vapors of the corresponding hydrohalic acid through powdered chromium or ferrochrome. The resulting gaseous chloride washes the chrome-plated product.
The process takes a long time - several hours. The layer applied in this way is much stronger connected to the base material than the one applied galvanically.
It all started with washing dishes...
In any analytical laboratory there is a large bottle with a dark liquid. This is a “chromic mixture” - a mixture of a saturated solution of potassium dichromate with concentrated sulfuric acid. Why is it needed?
There is always grease on a person's fingers, which easily transfers to glass. It is these deposits that the chrome mixture is designed to wash away. It oxidizes fat and removes its remains. But this substance must be handled with care. A few drops of a chrome mixture falling on a suit can turn it into something like a sieve: there are two substances in the mixture, and both are “robbers” - a strong acid and a strong oxidizing agent.
Chrome and wood
Even in our age of glass, aluminum, concrete and plastics, it is impossible not to recognize wood as an excellent building material. Its main advantage is its ease of processing, and its main disadvantages are its fire hazard, susceptibility to destruction by fungi, bacteria, and insects. Wood can be made more resistant by impregnating it with special solutions, which necessarily include chromates and dichromates, plus zinc chloride, copper sulfate, sodium arsenate and some other substances. Impregnation greatly increases the resistance of wood to fungi, insects, and flame.
Looking at the drawing
Illustrations in printed publications are made from cliches - metal plates on which this design (or rather, its mirror image) is engraved chemically or manually. Before the invention of photography, clichés were only engraved by hand; This is labor-intensive work that requires great skill.
But back in 1839, a discovery occurred that seemed to have nothing to do with printing. It was found that paper impregnated with sodium or potassium bichromate suddenly turns brown after being illuminated with bright light. Then it turned out that bichromate coatings on paper, after exposure, do not dissolve in water, but, when wetted, acquire a bluish tint. Printers took advantage of this property. The desired pattern was photographed on a plate with a colloidal coating containing dichromate. The illuminated areas did not dissolve during washing, but the unexposed areas dissolved, and a pattern remained on the plate from which it was possible to print.
Nowadays, other photosensitive materials are used in printing; the use of bichromate gels is being reduced. But we should not forget that chromium helped the “pioneers” of the photomechanical method in printing.
Similar documents
Electronic formula and oxidation state of chromium, its general content in the earth's crust and space. Methods for obtaining chromium, its physical and Chemical properties. Interaction of chromium with simple and complex substances. Features of application, main connections.
presentation, added 02/16/2013
Study of the physical and chemical properties of chromium, tungsten, molybdenum. Chromium oxide is the most stable chromium compound. Hydroxides, salts of oxygen-containing acids of elements of the sixth B group. Peroxides, carbides, nitrides, borides of elements of the sixth B group.
lecture, added 06/29/2011
Distribution of chromium in nature. Features of obtaining chromium and its compounds. Physical and chemical properties of chromium, its practical use in everyday life and industry. Inorganic chromium-based pigments, technology and methods for their production.
course work, added 06/04/2015
Preparation of pure chromium metal by electrolysis of aqueous solutions of chromium chloride. Basic physical and chemical properties of chromium. Characteristics of ammonium dichromate, potassium dichromide, their toxicity and application features. Preparation of chromic anhydride.
course work, added 01/07/2015
Features of the chemical properties of vanadium: discovery, use in chemical industry. Description of vanadium in its pure form (malleable metal of light gray color) and its compounds. Characteristics of the results of vanadium refining of steel and other metals.
abstract, added 01/23/2010
Chrome is a hard, shiny metal. Chromium is a component of stainless, acid-resistant, and heat-resistant steels. Chromium compounds. Oxygen is the most common element in the earth's crust. Production and properties of oxygen. Use of oxygen.
report, added 11/03/2006
Chemical properties of manganese and its compounds. Industrial production manganese History of the discovery of chromium, general information. Consumption standards for manganese and chromium, their biological role. The influence of a lack or excess of microelements on the human body.
abstract, added 01/20/2015
Oxidation states, electronic configurations, coordination numbers and geometry of chromium compounds. Characteristics of complex compounds. Multinuclear chromium complexes, their electronic compounds. Phosphorescent complexes, higher oxidation states of chromium.
course work, added 06/06/2010
General characteristics of manganese, its basic physical and chemical properties, history of discovery and modern achievements in research. Prevalence of this chemical element in nature, directions of its application in industry, production.
test, added 06/26/2013
Consideration of the structure and properties of electrodeposited chromium. Studying the technological features of chrome plating and carrying out its electrical, thermal, and structural calculations. Study of methods for purifying chromium-containing wastewater from galvanic production.
Hard metal of bluish-white color. Chrome is sometimes classified as a ferrous metal. This metal is capable of painting compounds in different colors, which is why it was named “chrome”, which means “paint”. Chromium is a microelement essential for normal development and functioning of the human body. Its most important biological role is the regulation of carbohydrate metabolism and blood glucose levels.
See also:
STRUCTURE

Depending on the types of chemical bonds - like all metals, chromium has a metallic type of crystal lattice, that is, the lattice nodes contain metal atoms.
Depending on the spatial symmetry - cubic, body-centered a = 0.28839 nm. A feature of chromium is a sharp change in its physical properties at a temperature of about 37°C. The crystal lattice of a metal consists of its ions and mobile electrons. Similarly, the chromium atom in its ground state has an electronic configuration. At 1830 °C it is possible to transform into a modification with a face-centered lattice, a = 3.69 Å.
PROPERTIES

Chromium has a Mohs hardness of 9, one of the hardest pure metals (second only to iridium, beryllium, tungsten and uranium). Very pure chrome can be machined quite well. Stable in air due to passivation. For the same reason, it does not react with sulfuric and nitric acids. At 2000 °C it burns to form green chromium(III) oxide Cr 2 O 3, which has amphoteric properties. When heated, it reacts with many non-metals, often forming compounds of non-stoichiometric composition: carbides, borides, silicides, nitrides, etc. Chromium forms numerous compounds in various oxidation states, mainly +2, +3, +6. Chrome has all the properties characteristic of metals - it conducts heat and electricity well, and has the luster characteristic of most metals. It is antiferromagnetic and paramagnetic, that is, at a temperature of 39 °C it changes from a paramagnetic state to an antiferromagnetic state (Néel point).
RESERVES AND PRODUCTION

The largest chromium deposits are located in South Africa (1st place in the world), Kazakhstan, Russia, Zimbabwe, and Madagascar. There are also deposits in Turkey, India, Armenia, Brazil, and the Philippines.nThe main deposits of chromium ores in the Russian Federation are known in the Urals (Don and Saranovskoe). Explored reserves in Kazakhstan amount to over 350 million tons (2nd place in the world). Chromium is found in nature mainly in the form of chromium iron ore Fe(CrO 2) 2 (iron chromite). Ferrochrome is obtained from it by reduction in electric furnaces with coke (carbon). To obtain pure chromium, the reaction is carried out as follows:
1) iron chromite is fused with sodium carbonate (soda ash) in air;
2) dissolve sodium chromate and separate it from iron oxide;
3) convert the chromate to dichromate, acidifying the solution and crystallizing the dichromate;
4) pure chromium oxide is obtained by reducing sodium dichromate with coal;
5) metallic chromium is obtained using aluminothermy;
6) using electrolysis, electrolytic chromium is obtained from a solution of chromic anhydride in water containing the addition of sulfuric acid.
ORIGIN

The average content of Chromium in the earth's crust (clarke) is 8.3·10 -3%. This element is probably more characteristic of the Earth's mantle, since ultramafic rocks, which are believed to be closest in composition to the Earth's mantle, are enriched in Chromium (2·10 -4%). Chromium forms massive and disseminated ores in ultramafic rocks; The formation of the largest chromium deposits is associated with them. In basic rocks, the Chromium content reaches only 2·10 -2%, in acidic rocks - 2.5·10 -3%, in sedimentary rocks (sandstones) - 3.5·10 -3%, in clay shales - 9·10 -3 %. Chromium is a relatively weak aquatic migrant; Chromium content in sea water is 0.00005 mg/l.
In general, Chromium is a metal in the deep zones of the Earth; stony meteorites (analogues of the mantle) are also enriched in Chromium (2.7·10 -1%). Over 20 chromium minerals are known. Only chrome spinels (up to 54% Cr) are of industrial importance; in addition, Chromium is contained in a number of other minerals, which often accompany chromium ores, but are not of practical value themselves (uvarovite, volkonskoite, kemerite, fuchsite).
There are three main chromium minerals: magnochromite (Mg, Fe)Cr 2 O 4 , chrompicotite (Mg, Fe)(Cr, Al) 2 O 4 and aluminochromite (Fe, Mg)(Cr, Al) 2 O 4 . By appearance they are indistinguishable and are inaccurately called "chromites".
APPLICATION

Chromium is an important component in many alloy steels (in particular stainless steels), as well as in a number of other alloys. The addition of chromium significantly increases the hardness and corrosion resistance of alloys. The use of Chrome is based on its heat resistance, hardness and corrosion resistance. Most of all, Chromium is used for smelting chromium steels. Aluminum- and silicothermic chromium is used for smelting nichrome, nimonic, other nickel alloys and stellite.
A significant amount of Chromium is used for decorative corrosion-resistant coatings. Powdered Chromium is widely used in the production of metal-ceramic products and materials for welding electrodes. Chromium, in the form of Cr 3+ ion, is an impurity in ruby, which is used as a gemstone and laser material. Chromium compounds are used to etch fabrics during dyeing. Some Chromium salts are used as a component of tanning solutions in the leather industry; PbCrO 4 , ZnCrO 4 , SrCrO 4 - like art paints. Chromium-magnesite refractory products are made from a mixture of chromite and magnesite.
Used as wear-resistant and beautiful galvanic coatings (chrome plating).
Chromium is used for the production of alloys: chromium-30 and chromium-90, which are indispensable for the production of nozzles for powerful plasma torches and in the aerospace industry.
Chrome (eng. Chromium) - Cr
- Designation - Cr (Chromium);
- Period - IV;
- Group - 6 (VIb);
- Atomic mass - 51.9961;
- Atomic number - 24;
- Atomic radius = 130 pm;
- Covalent radius = 118 pm;
- Electron distribution - 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1 ;
- melting temperature = 1857°C;
- boiling point = 2672°C;
- Electronegativity (according to Pauling/according to Alpred and Rochow) = 1.66/1.56;
- Oxidation state: +6, +3, +2, 0;
- Density (no.) = 7.19 g/cm3;
- Molar volume = 7.23 cm 3 /mol.
Chromium (color, paint) was first found at the Berezovsky gold deposit (Middle Urals), the first mentions date back to 1763; in his work “The First Foundations of Metallurgy” M.V. Lomonosov calls it “red lead ore”.

Rice. Structure of the chromium atom.
The electronic configuration of the chromium atom is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1 (see Electronic structure of atoms). In education chemical bonds with other elements, 1 electron located on the outer 4s level + 5 electrons of the 3d sublevel (6 electrons in total) can participate, so in compounds chromium can take oxidation states from +6 to +1 (the most common are +6, +3, +2). Chromium is a chemically inactive metal; it reacts with simple substances only at high temperatures.
Physical properties of chromium:
- bluish-white metal;
- very hard metal (in the presence of impurities);
- fragile when n. y.;
- plastic (in its pure form).
Chemical properties of chromium
- at t=300°C reacts with oxygen:
4Cr + 3O 2 = 2Cr 2 O 3; - at t>300°C reacts with halogens, forming mixtures of halides;
- at t>400°C reacts with sulfur to form sulfides:
Cr + S = CrS; - at t=1000°C finely ground chromium reacts with nitrogen, forming chromium nitride (a semiconductor with high chemical stability):
2Cr + N 2 = 2CrN; - reacts with dilute hydrochloric and sulfuric acids to release hydrogen:
Cr + 2HCl = CrCl 2 + H 2;
Cr + H 2 SO 4 = CrSO 4 + H 2; - warm concentrated nitrogen and sulfuric acid dissolve chromium.
With concentrated sulfuric and nitric acid at no. chromium does not react, and chromium also does not dissolve in aqua regia; it is noteworthy that pure chromium does not react even with dilute sulfuric acid; the reason for this phenomenon has not yet been established. During long-term storage in concentrated nitric acid, chromium becomes covered with a very dense oxide film (passivates) and stops reacting with dilute acids.
Chromium compounds
It was already said above that the “favorite” oxidation states of chromium are +2 (CrO, Cr(OH) 2), +3 (Cr 2 O 3, Cr(OH) 3), +6 (CrO 3, H 2 CrO 4 ).
Chrome is chromophore, i.e., an element that gives color to the substance in which it is contained. For example, in the oxidation state +3, chromium gives a purple-red or green color (ruby, spinel, emerald, garnet); in the oxidation state +6 - yellow-orange color (crocoite).
In addition to chromium, chromophores also include iron, nickel, titanium, vanadium, manganese, cobalt, copper - all these are d-elements.
The color of common compounds that include chromium:
- chromium in oxidation state +2:
- chromium oxide CrO - red;
- chromium fluoride CrF 2 - blue-green;
- chromium chloride CrCl 2 - has no color;
- chromium bromide CrBr 2 - has no color;
- Chromium iodide CrI 2 - red-brown.
- chromium in oxidation state +3:
- Cr 2 O 3 - green;
- CrF 3 - light green;
- CrCl 3 - violet-red;
- CrBr 3 - dark green;
- CrI 3 - black.
- chromium in oxidation state +6:
- CrO 3 - red;
- potassium chromate K 2 CrO 4 - lemon yellow;
- ammonium chromate (NH 4) 2 CrO 4 - golden yellow;
- calcium chromate CaCrO 4 - yellow;
- Lead chromate PbCrO 4 - light brown-yellow.
Chromium oxides:
- Cr +2 O - basic oxide;
- Cr 2 +3 O 3 - amphoteric oxide;
- Cr +6 O 3 - acidic oxide.
Chromium hydroxides:
- ".
Application of chromium
- as a alloying additive in the smelting of heat-resistant and corrosion-resistant alloys;
- for chrome plating of metal products in order to give them high corrosion resistance, abrasion resistance and a beautiful appearance;
- chromium-30 and chromium-90 alloys are used in plasma torch nozzles and in the aviation industry.
Chromium is an element of the side subgroup of the 6th group of the 4th period of the periodic system of chemical elements of D.I. Mendeleev, with atomic number 24. It is designated by the symbol Cr (lat. Chromium). The simple substance chromium is a hard metal of a bluish-white color.
Chemical properties of chromium
Under normal conditions, chromium reacts only with fluorine. At high temperatures (above 600°C) it interacts with oxygen, halogens, nitrogen, silicon, boron, sulfur, phosphorus.
4Cr + 3O 2 – t° →2Cr 2 O 3
2Cr + 3Cl 2 – t° → 2CrCl 3
2Cr + N 2 – t° → 2CrN
2Cr + 3S – t° → Cr 2 S 3
When heated, it reacts with water vapor:
2Cr + 3H 2 O → Cr 2 O 3 + 3H 2
Chromium dissolves in dilute strong acids(HCl, H2SO4)
In the absence of air, Cr 2+ salts are formed, and in air, Cr 3+ salts are formed.
Cr + 2HCl → CrCl 2 + H 2
2Cr + 6HCl + O 2 → 2CrCl 3 + 2H 2 O + H 2
The presence of a protective oxide film on the surface of the metal explains its passivity in relation to concentrated solutions of acids - oxidizers.
Chromium compounds
Chromium(II) oxide and chromium(II) hydroxide are basic in nature.
Cr(OH) 2 + 2HCl → CrCl 2 + 2H 2 O
Chromium (II) compounds are strong reducing agents; transform into chromium (III) compounds under the influence of atmospheric oxygen.
2CrCl 2 + 2HCl → 2CrCl 3 + H 2
4Cr(OH) 2 + O 2 + 2H 2 O → 4Cr(OH) 3
Chromium oxide (III) Cr 2 O 3 is a green, water-insoluble powder. Can be obtained by calcination of chromium(III) hydroxide or potassium and ammonium dichromates:
2Cr(OH) 3 – t° → Cr 2 O 3 + 3H 2 O
4K 2 Cr 2 O 7 – t° → 2Cr 2 O 3 + 4K 2 CrO 4 + 3O 2
(NH 4) 2 Cr 2 O 7 – t° → Cr 2 O 3 + N 2 + 4H 2 O (volcano reaction)
Amphoteric oxide. When Cr 2 O 3 is fused with alkalis, soda and acid salts, chromium compounds with an oxidation state of (+3) are obtained:
Cr 2 O 3 + 2NaOH → 2NaCrO 2 + H 2 O
Cr 2 O 3 + Na 2 CO 3 → 2NaCrO 2 + CO 2
When fused with a mixture of alkali and oxidizing agent, chromium compounds are obtained in the oxidation state (+6):
Cr 2 O 3 + 4KOH + KClO 3 → 2K 2 CrO 4 + KCl + 2H 2 O
Chromium (III) hydroxide C r (OH) 3 . Amphoteric hydroxide. Gray-green, decomposes when heated, losing water and forming green metahydroxide CrO(OH). Does not dissolve in water. Precipitates from solution as a gray-blue and bluish-green hydrate. Reacts with acids and alkalis, does not interact with ammonia hydrate.
It has amphoteric properties - it dissolves in both acids and alkalis:
2Cr(OH) 3 + 3H 2 SO 4 → Cr 2 (SO 4) 3 + 6H 2 O Cr(OH) 3 + ZH + = Cr 3+ + 3H 2 O
Cr(OH) 3 + KOH → K, Cr(OH) 3 + ZON - (conc.) = [Cr(OH) 6 ] 3-
Cr(OH) 3 + KOH → KCrO 2 + 2H 2 O Cr(OH) 3 + MOH = MSrO 2 (green) + 2H 2 O (300-400 °C, M = Li, Na)
Cr(OH) 3 →(120 o C – H 2 O) CrO(OH) →(430-1000 0 C –H 2 O) Cr2O3
2Cr(OH) 3 + 4NaOH (conc.) + ZN 2 O 2 (conc.) = 2Na 2 CrO 4 + 8H 2 0
Receipt: precipitation with ammonia hydrate from a solution of chromium(III) salts:
Cr 3+ + 3(NH 3 H 2 O) = WITHr(OH) 3 ↓+ ЗNН 4+
Cr 2 (SO 4) 3 + 6NaOH → 2Cr(OH) 3 ↓+ 3Na 2 SO 4 (in excess alkali - the precipitate dissolves)
Chromium (III) salts have a purple or dark green color. Their chemical properties resemble colorless aluminum salts.
Cr(III) compounds can exhibit both oxidizing and reducing properties:
Zn + 2Cr +3 Cl 3 → 2Cr +2 Cl 2 + ZnCl 2
2Cr +3 Cl 3 + 16NaOH + 3Br 2 → 6NaBr + 6NaCl + 8H 2 O + 2Na 2 Cr +6 O 4
Hexavalent chromium compounds
Chromium(VI) oxide CrO 3 - bright red crystals, soluble in water.
Obtained from potassium chromate (or dichromate) and H 2 SO 4 (conc.).
K 2 CrO 4 + H 2 SO 4 → CrO 3 + K 2 SO 4 + H 2 O
K 2 Cr 2 O 7 + H 2 SO 4 → 2CrO 3 + K 2 SO 4 + H 2 O
CrO 3 is an acidic oxide, with alkalis it forms yellow chromates CrO 4 2-:
CrO 3 + 2KOH → K 2 CrO 4 + H 2 O
In an acidic environment, chromates turn into orange dichromates Cr 2 O 7 2-:
2K 2 CrO 4 + H 2 SO 4 → K 2 Cr 2 O 7 + K 2 SO 4 + H 2 O
In an alkaline environment, this reaction proceeds in the opposite direction:
K 2 Cr 2 O 7 + 2KOH → 2K 2 CrO 4 + H 2 O
Potassium dichromate is an oxidizing agent in an acidic environment:
K 2 Cr 2 O 7 + 4H 2 SO 4 + 3Na 2 SO 3 = Cr 2 (SO 4) 3 + 3Na 2 SO 4 + K 2 SO 4 + 4H 2 O
K 2 Cr 2 O 7 + 4H 2 SO 4 + 3NaNO 2 = Cr 2 (SO 4) 3 + 3NaNO 3 + K 2 SO 4 + 4H 2 O
K 2 Cr 2 O 7 + 7H 2 SO 4 + 6KI = Cr 2 (SO 4) 3 + 3I 2 + 4K 2 SO 4 + 7H 2 O
K 2 Cr 2 O 7 + 7H 2 SO 4 + 6FeSO 4 = Cr 2 (SO 4) 3 + 3Fe 2 (SO 4) 3 + K 2 SO 4 + 7H 2 O
Potassium chromate K 2 Cr O 4 . Oxosol. Yellow, non-hygroscopic. Melts without decomposition, thermally stable. Very soluble in water ( yellow the color of the solution corresponds to the CrO 4 2- ion), slightly hydrolyzes the anion. In an acidic environment it turns into K 2 Cr 2 O 7 . Oxidizing agent (weaker than K 2 Cr 2 O 7). Enters into ion exchange reactions.
Qualitative reaction on the CrO 4 2- ion - the precipitation of a yellow precipitate of barium chromate, which decomposes in a strongly acidic environment. It is used as a mordant for dyeing fabrics, a leather tanning agent, a selective oxidizing agent, and a reagent in analytical chemistry.
Equations of the most important reactions:
2K 2 CrO 4 +H 2 SO 4(30%)= K 2 Cr 2 O 7 +K 2 SO 4 +H 2 O
2K 2 CrO 4 (t) +16HCl (concentration, horizon) = 2CrCl 3 +3Cl 2 +8H 2 O+4KCl
2K 2 CrO 4 +2H 2 O+3H 2 S=2Cr(OH) 3 ↓+3S↓+4KOH
2K 2 CrO 4 +8H 2 O+3K 2 S=2K[Cr(OH) 6 ]+3S↓+4KOH
2K 2 CrO 4 +2AgNO 3 =KNO 3 +Ag 2 CrO 4(red) ↓
Qualitative reaction:
K 2 CrO 4 + BaCl 2 = 2KCl + BaCrO 4 ↓
2BaCrO 4 (t) + 2HCl (dil.) = BaCr 2 O 7 (p) + BaC1 2 + H 2 O
Receipt: sintering of chromite with potash in air:
4(Cr 2 Fe ‖‖)O 4 + 8K 2 CO 3 + 7O 2 = 8K 2 CrO 4 + 2Fe 2 O 3 + 8СO 2 (1000 °C)
Potassium dichromate K 2 Cr 2 O 7 . Oxosol. Technical name chrome peak. Orange-red, non-hygroscopic. Melts without decomposition, and decomposes upon further heating. Very soluble in water ( orange The color of the solution corresponds to the Cr 2 O 7 2- ion. In an alkaline environment it forms K 2 CrO 4 . A typical oxidizing agent in solution and during fusion. Enters into ion exchange reactions.
Qualitative reactions- blue color of an ethereal solution in the presence of H 2 O 2, blue color of an aqueous solution under the action of atomic hydrogen.
It is used as a leather tanning agent, a mordant for dyeing fabrics, a component of pyrotechnic compositions, a reagent in analytical chemistry, a metal corrosion inhibitor, in a mixture with H 2 SO 4 (conc.) - for washing chemical dishes.
Equations of the most important reactions:
4K 2 Cr 2 O 7 =4K 2 CrO 4 +2Cr 2 O 3 +3O 2 (500-600 o C)
K 2 Cr 2 O 7 (t) +14HCl (conc) = 2CrCl 3 +3Cl 2 +7H 2 O+2KCl (boiling)
K 2 Cr 2 O 7 (t) +2H 2 SO 4(96%) ⇌2KHSO 4 +2CrO 3 +H 2 O (“chromium mixture”)
K 2 Cr 2 O 7 +KOH (conc) =H 2 O+2K 2 CrO 4
Cr 2 O 7 2- +14H + +6I - =2Cr 3+ +3I 2 ↓+7H 2 O
Cr 2 O 7 2- +2H + +3SO 2 (g) = 2Cr 3+ +3SO 4 2- +H 2 O
Cr 2 O 7 2- +H 2 O +3H 2 S (g) =3S↓+2OH - +2Cr 2 (OH) 3 ↓
Cr 2 O 7 2- (conc.) +2Ag + (dil.) =Ag 2 Cr 2 O 7 (red) ↓
Cr 2 O 7 2- (dil.) +H 2 O +Pb 2+ =2H + + 2PbCrO 4 (red) ↓
K 2 Cr 2 O 7(t) +6HCl+8H 0 (Zn)=2CrCl 2(syn) +7H 2 O+2KCl
Receipt: treatment of K 2 CrO 4 with sulfuric acid:
2K 2 CrO 4 + H 2 SO 4 (30%) = K 2Cr 2 O 7 + K 2 SO 4 + H 2 O

The content of the article
CHROMIUM– (Chromium) Cr, chemical element 6(VIb) groups Periodic table. Atomic number 24, atomic mass 51.996. There are 24 known isotopes of chromium from 42 Cr to 66 Cr. The isotopes 52 Cr, 53 Cr, 54 Cr are stable. Isotopic composition of natural chromium: 50 Cr (half-life 1.8 10 17 years) – 4.345%, 52 Cr – 83.489%, 53 Cr – 9.501%, 54 Cr – 2.365%. The main oxidation states are +3 and +6.
In 1761, chemistry professor at St. Petersburg University Johann Gottlob Lehmann, at the eastern foot of the Ural Mountains at the Berezovsky mine, discovered a wonderful red mineral, which, when crushed into powder, gave a bright yellow color. In 1766 Lehman brought samples of the mineral to St. Petersburg. Having treated the crystals with hydrochloric acid, he obtained a white precipitate, in which he discovered lead. Lehmann called the mineral Siberian red lead (plomb rouge de Sibérie); it is now known that it was crocoite (from the Greek “krokos” - saffron) - a natural lead chromate PbCrO 4.
The German traveler and naturalist Peter Simon Pallas (1741–1811) led an expedition of the St. Petersburg Academy of Sciences to the central regions of Russia and in 1770 visited the Southern and Middle Urals, including the Berezovsky mine and, like Lehmann, became interested in crocoite. Pallas wrote: “This amazing red lead mineral is not found in any other deposit. When ground into powder it turns yellow and can be used in artistic miniatures.” Despite the rarity and difficulty of delivering crocoite from the Berezovsky mine to Europe (it took almost two years), the use of the mineral as a coloring agent was appreciated. In London and Paris at the end of the 17th century. all noble persons rode in carriages painted with finely ground crocoite; in addition, the best examples of Siberian red lead replenished the collections of many mineralogical cabinets in Europe.
In 1796, a sample of crocoite came to the professor of chemistry at the Paris Mineralogical School, Nicolas-Louis Vauquelin (1763–1829), who analyzed the mineral, but found nothing in it except oxides of lead, iron and aluminum. Continuing his research on Siberian red lead, Vaukelin boiled the mineral with a solution of potash and, after separating the white precipitate of lead carbonate, obtained a yellow solution of an unknown salt. When treated with lead salt, a yellow precipitate was formed, with mercury salt, a red one, and when tin chloride was added, the solution became green. By decomposing crocoite with mineral acids, he obtained a solution of “red lead acid,” the evaporation of which gave ruby-red crystals (it is now clear that it was chromic anhydride). Having calcined them with coal in a graphite crucible, after the reaction I discovered many fused gray needle-shaped crystals of a metal unknown to that time. Vaukelin noted the high refractoriness of the metal and its resistance to acids.
Vaukelin named the new element chromium (from the Greek crwma - color, color) due to the many multi-colored compounds it forms. Based on his research, Vauquelin was the first to state that the emerald color of some precious stones is explained by the admixture of chromium compounds in them. For example, natural emerald is a deep green colored beryl in which aluminum is partially replaced by chromium.
Most likely, Vauquelin obtained not pure metal, but its carbides, as evidenced by the needle-shaped shape of the resulting crystals, but the Paris Academy of Sciences nevertheless registered the discovery of a new element, and now Vauquelin is rightly considered the discoverer of element No. 24.
Yuri Krutyakov
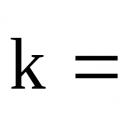 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe