Rate constant of a chemical reaction. Reaction rate constant Guidelines for laboratory work
According to the law of mass action, the rate of a simple reaction is equal to
Reaction rate constant k
-
coefficient of proportionality between the rate of a chemical reaction and the product of the concentrations of the reacting substances:  . The rate constant is numerically equal to the rate of a chemical reaction when the concentrations of all reactants are equal to unity: W=k at C A =C B =1. If the reaction of A with B is complex in its mechanism (it involves active intermediate products, a catalyst, etc.), it obeys the equation
. The rate constant is numerically equal to the rate of a chemical reaction when the concentrations of all reactants are equal to unity: W=k at C A =C B =1. If the reaction of A with B is complex in its mechanism (it involves active intermediate products, a catalyst, etc.), it obeys the equation  , then k is called effective reaction rate constant; IUPAC recommends calling k in this case reaction rate coefficient. Often the rate of a complex reaction does not obey a power equation, but is expressed by another dependence, for example v=k 1 C 1 C 2 (1+k 2 C 2) –1. Then k 1 and k 2 are called coefficients in the equation for the reaction rate.
, then k is called effective reaction rate constant; IUPAC recommends calling k in this case reaction rate coefficient. Often the rate of a complex reaction does not obey a power equation, but is expressed by another dependence, for example v=k 1 C 1 C 2 (1+k 2 C 2) –1. Then k 1 and k 2 are called coefficients in the equation for the reaction rate.
Often the reaction is carried out under conditions where the concentrations of all reagents, except one, are taken in excess and practically do not change during the experiment. In this case
 ,
,
and coefficient k obs. =
k  called effective or observed reaction rate constant at C B >>C A . For the case n A =1, such a coefficient is often called the pseudo-first order reaction rate coefficient. The reaction rate constant of order n has the dimension: (time) –1 (concentration) –(n –1) . The numerical value depends on the units chosen to measure time and concentration.
called effective or observed reaction rate constant at C B >>C A . For the case n A =1, such a coefficient is often called the pseudo-first order reaction rate coefficient. The reaction rate constant of order n has the dimension: (time) –1 (concentration) –(n –1) . The numerical value depends on the units chosen to measure time and concentration.
When calculating the rate constant of a simple reaction, it is necessary to take into account two circumstances: remember which reagent the reaction rate is measured from and what the stoichiometric coefficient and reaction order for this reagent are. For example, the reaction of a 2,4,6-trialkylphenoxy radical with hydroperoxide occurs in two successive stages:
PhО +ROOH→PhOH+RO 2
PhO +RO 2 →ROOPhO
The stoichiometric equation is 2PhO +ROOH=PhOH+ROOPhO, but since the first stage determines the reaction rate, W ROOH =k and W PhO =2k.
Thus, the coefficients in the kinetic and stoichiometric equations for the phenoxyl radical do not coincide here: the reaction order in PhO is 1, and the stoichiometric coefficient for PhO is 2.
Methods for calculating the rate constant of a chemical reaction. According to the kinetic curve. If n = 1, then k=t –1 ln 10 lg (C Ao /C A). If the total reaction order is n, and the reaction order for a given component is 1, and all reagents except A are taken in excess, then
 .
.
For the reaction A+B→products k are found from the equation

When calculating the rate constant from the integral kinetic curve in general form, the task is to determine k in the equation f(x)= –k`t (x is the relative concentration of the reagent).
For a 1st order reaction f(x)=ln x, k`=k; for a 2nd order reaction f(x)=x –1 –1, k=C o k, etc. From the experiment we obtain a series of values (t 1, x 1), (t 2, x 2), ..., (t n, x n). A straight line drawn in coordinates f(x)–t must satisfy the condition i =f(x i)+kt i, Σ i =0. It follows that k= Σf(x i)/Σt i.
According to the half-life period. The half-life is uniquely related to the rate constant and the initial concentration of the reagent, which allows us to calculate k. So, for a first-order reaction k=ln 2/τ 1/2, for a second-order reaction k=C o –1 τ 1/2, etc.
According to the initial reaction rate. Since at the initial moment of time the consumption of reagents is insignificant,
 And
And 
By the change in reaction rate over time. By measuring the concentrations of the reactants at time t` and t`` (С` and С``), we can calculate the average reaction rate and find k, with ν=1 we have
 ,
,
 ,
,
 .
.
Special methods for processing kinetic curves. If the kinetics of a reaction is recorded by a change in any physical property of the system x (optical density, electrical conductivity, etc.) associated with the concentration of the reactant C so that at C=C o , x=x o , and at C=0 , x=x ∞ , then k can be determined from the kinetic curve x(t) using the following methods:
Guggenheim method(for first order reactions). Measure x i at time t i and x 1 ` at time t i +, etc. From the graph lg (х i –х i`)–t i find k:
log (x i –x i `)=log[(x o –x ∞)(1–e – k )]–0.43kt i .
Mangelsdorff method(for first order reactions). Measurements are carried out as in the Guggenheim method, but the graph is plotted in coordinates x i ` – x i:
x i `=x i e –k +x ∞ (1–e –k ),
the slope of the straight line is equal to e – k , the intercept on the ordinate axis is equal to x ∞ (1 – e – k ).
Rosevery method(for second order reactions). The x parameter is measured at times t 1, t 2, t 3 separated by a constant time interval . The rate constant is found from the equation:
 .
.
Section 5. KINETICS OF CHEMICAL REACTIONS AND CATALYSIS.
Thermodynamically possible reactions are not always carried out in reality. This is due to the fact that in thermodynamics there is no time parameter, so it does not answer how soon a given state will occur. Determining the conditions under which thermodynamically possible reactions will proceed at a sufficient rate is one of the main problems of chemical kinetics. In kinetics, the time factor is introduced, which is not considered in thermodynamics.
Chemical kinetics - this is the doctrine of the regularity of the course of a chemical process over time or the doctrine of the mechanisms and speed of chemical reactions.
The set of stages that make up a chemical reaction is called mechanism or scheme of a chemical reaction.
The rate of a chemical reaction.
Under the speed of a chemical reaction understand the change in the number of moles of reacting substances per unit time per unit volume.
There are average speeds ( u cf) and true ( u).
average speed - change in the concentration of reacting substances over a given period of time:
u av = ± (n 2 – n 1) / V(t 2 - t 1) = ± Dn / V Δt = ± Δс / Δt.
The ratio Δс/Δt can be either positive or negative. The speed can be measured by monitoring the decrease in the concentration of the original compound, then we put a minus sign in front of the ratio, since the speed is always a positive value. If the speed is expressed through the concentration of the receiving substance, then the plus sign:
- Δс А / Δt= + Δс В /Δt.
You can relate the change in concentration to an infinitesimal period of time (t 2 -t 1 → 0), defining true reaction speed at the moment as a derivative of concentration over time (u = ±dс/dt).
- dc A /dt = + dc B /dt
Dependence of reaction rate on concentration.
The main postulate of chemical kinetics is the law of mass action established by Guldberg and Vahe. Consider the chemical reaction:
m 1 A + m 2 B → m 3 C + m 4 D.
The equation that describes the dependence of the rate of a chemical reaction on the concentration of the components of the reaction mixture is called kinetic equation of a chemical reaction.
The kinetic equation of the reaction under consideration:
u = kс A m 1 ´s B m 2 ,
where k is the proportionality coefficient (speed constant).
Law of mass action: the rate of a chemical reaction at each moment of time is directly proportional to the product of the concentrations of the reacting substances at a given time in powers corresponding to the stoichiometric reaction coefficients (in the simplest case).
In most cases, it is not the speed that is calculated, but the rate constant. If c A = c B = 1 mol/l, then u = k.
Physical meaning of the rate constant: the rate constant of a chemical reaction is numerically equal to the reaction rate, provided that the concentrations of the reactants are constant and equal to unity. The rate constant is independent of concentration and depends on temperature, the nature of the solvent and the presence of a catalyst.
All reactions are kinetically two-way or kinetically reversible. A chemical reaction is reversible when the reaction products can react with each other to form the starting materials. In practice, the reverse reaction can be so slow compared to the direct one that, with any reasonable accuracy, the reversibility of the reaction can be neglected and the reaction can be considered irreversible or one-sided. Strictly speaking, any chemical reactions are reversible:
m 1 A 4 +m 2 B « m 3 C+m 4 D
u = u 1 - u 2 = k 1 s A m 1 ´s B m 2 - k 2 s WITH m 3´s D m 4,
At the moment of chemical equilibrium u 1 = u 2 , those
k 1 s A m 1 ´s B m 2 = k 2 s WITH m 3´s D m 4,
TO =k 1 / k 2=With WITH m 3´ With D m 4/ With A m 1´ With B m 2
where K is the chemical equilibrium constant, equal to the ratio of the rate constant of the forward reaction to the rate constant of the reverse reaction.
Classification of reactions by molecularity and order.
When studying kinetics, chemical reactions differ in molecularity and order.
Molecularity of the reaction is determined by the number of molecules participating simultaneously in the stage that determines the rate of the entire reaction (the slowest). On this basis, reactions are divided into mono-, bi- and trimolecular. Reactions of higher molecularity are practically unknown, since the probability of four molecules meeting is negligible.
Order of reactions is determined by the sum of the exponents of the concentrations in the expression of the law of mass action. A distinction is made between a complete (general) reaction order and a partial one (for each reagent). The sum of the exponents in which the concentrations of all starting substances enter the kinetic equation determines the overall order. There are reactions of zero, first, second, third and fractional orders.
The coincidence of molecularity with order is observed only in the simplest cases, when the reaction proceeds in one stage:
2NO + H 2 ↔ N 2 O + H 2 O,
general order - 3, molecularity - 3.
5.3.1. First order one-way reaction equation.
Consider the chemical reaction: A → B.
u = kс = - dс/dt.
Let's separate the variables: -dс/с = k dt, integrate
Lnc = kt + const,
if τ = 0 (initial moment of the reaction), then const = ln c 0, i.e.
Ln с = kt - ln с 0,
ln s 0 - ln s = kt or ln s 0 / s = kt,
k = (1/t)´ ln s 0 /s.
Let us denote x - the degree of conversion of the starting substance: x = c 0 – c.
k = (1/t) ´ln s 0 /(s 0 - x),
dimension - [time -1 ].
The rate constant of a first-order reaction is independent of concentration. You can substitute the concentration (mol/l) or the number of moles into the resulting equation. Instead of “c 0” and “(c 0 - x)” you can substitute any values proportional to the concentration (electrical conductivity, density, viscosity, etc.).
To characterize the rate of a first-order reaction, along with the rate constant, a quantity called half-life is often used.
Half life(t 1/2)- the period of time during which half of the taken amount of substance reacts:
t 1/2 = (1/k)´ ln s 0 /(s 0 - x), where x = 1/2s 0.
We get:
t 1/2 = ln2/k = 0.693/k.
The half-life does not depend on the initial concentrations, but depends on the rate constant, i.e. it is a characteristic of a first order reaction.
First-order reactions include radioactive decay, isomerization, and most hydrolysis reactions. When there is a large excess of one of the reactants compared to the others, its concentration remains almost constant during the reaction. In this case, the order of the reaction will be one less than would be expected from the stoichiometric equation.
Bimolecular reactions in which the reaction order, due to an excess of one of the reactants, decreases by one, are called pseudomolecular.
Example, reaction of hydrolytic decomposition of sugar in a dilute aqueous solution (sugar inversion):
C 12 H 22 O 11 + H 2 O ↔ C 12 H 22 O 11 + C 12 H 22 O 11
sucrose glucose fructose
u = k[sucrose]´,
u = k* [sucrose], where k* = k´.
This is an example of a pseudo-first order reaction.
Second order one-way reaction equation.
A + B → C + D
Example: H 2 + J 2 = 2HJ;
2HJ = H 2 + J 2 ;
CH 3 COOC 2 H 5 + NaOH = CH 3 COONa + C 2 H 5 OH.
Dс/dt = ks 1 ´с 2
When с 1 = с 2 we get: -dс/dt =kс 2 or -dс/с 2 = k dt. Let's integrate:
1/s = kt + const.
At t = 0 → const = 1/s 0.
1/s - 1/s 0 = kt or (s 0 – s)/s´s 0 = kt;
c 0 - c = x, where x is the degree of conversion; c = c 0 - x;
x / s 0 (s 0 - s) = kt;
k = (1/ t)´,
dimension - [time -1 ´concentration -1 ].
The rate constant of a second-order reaction depends on the dimension of concentration.
Half-life: t 1/2 = (1/ k), where x = 1/2s 0, then
t 1/2 = 1/ ks 0 .
The half-life depends on the initial concentration and is not a characteristic of a second-order reaction.
Zero order reaction equation.
The rate of a chemical reaction does not depend on the concentration of the reactants (reactions at the interface, the limiting process is the diffusion process):
Dc/dt = ks 0 ; or -dс = k dt.
We integrate, we get: -с = kt + const.
At t = 0 → const = -с 0. We get: -с = kt - с 0;
k = (c 0 - c) /t = x/t,
dimension - [concentration ´time -1].
Half life:
t 1/2 = c 0 /2k
Methods for determining reaction order and rate constant.
In the kinetics of reactions of simple and complex types, the following problems are mainly solved:
1. Direct task: the order of the reaction and its rate constant are known. It is required to find the concentration of any of the starting substances or reaction products at a certain point in time or to find the time during which the concentration of any of the reactants or reaction products reaches a certain value.
2. Inverse problem: experimental data on the kinetics of a previously unstudied reaction were obtained. It is required to determine the reaction order and rate constant.
To determine the reaction order, it is necessary to have experimental data on changes in the concentration of reactants over time:
| from 0 | from 1 | from 2 | from 3 | from 4 | ….. |
| t 0 | t 1 | t 2 | t 3 | t 4 | ….. |
1. Method for selecting equations.
The method consists of substituting experimental data on the concentration of substances for each moment from the beginning of the reaction into kinetic equations of various orders (this technique does not give anything if the reaction order exceeds 3 or is fractional):
k = (s 0 - s) /t = x/t(zero order);
k = (1/t) lnc 0 /s(first order);
k = (1/t) x /s 0 s ( second order).
The reaction order will correspond to the kinetic equation for which, at different initial concentrations of the starting substances and at different times at a given temperature, the rate constant will be a constant value.
2. Graphical integral methods.
 |  |
zero order: first order second order
Rice. 5.1. Concentration changes over time for reactions
different orders.
Find such a function of concentration, plotting it on the graph depending on time to obtain a straight line (Fig. 5.1.).
3. By half-life.
According to the dependence of the half-life on the initial concentration:
zero order: t 1/2 = c 0 /2k;
first order: t 1/2 = 0.693/ k;
second order: t 1/2 = 1 / ks 0.
In general:
t 1/2 ≈ 1 /k s 0 n-1.
Experiments are carried out at two different initial concentrations (from 0)’ and (from 0)’:
(t 1/2) ’ = 1 /k (s 0) 1 n-1 (1)
(t 1/2)” = 1 /k (s 0) 2 n-1 (2)
Let's divide (1) by (2):
(t 1/2) ’ / (t 1/2)” = (s 0) 2 n-1 / (s 0) 1 n-1 .
Let's take a logarithm:
log(t 1/2) ’ / (t 1/2)” = (n-1) ´ log[(s 0) 2 /(s 0) 1 ],
n = 1 + / .
4.Differential method (van't Hoff method).
The dependence of the reaction rate on concentration is used, provided that the concentrations of all starting substances are equal (Fig. 5.2.): u = kс n. Let's take a logarithm of this expression: lgu = lgk + nlgс.

Rice. 5.2. Dependence of reaction rate on concentration.
5. Integral van't Hoff method (according to the dependence of the reaction rate on the initial concentration at the first moments of time - 10-15 s).
u = k (c 0 - x) n = k c 0 n ,
Since at the first moment of time x ≈ 0.
The experiment is carried out with different initial concentrations.
u 1 = k c 1 n (1)
u 2 = k c 2 n (2)
We divide equation (1) by equation (2): u 1 / u 2 = (c 1 / c 2) n.
Let's take logarithms:
n = (lgu 1 - lgu 2) / (lgс 1 -lgс 2),
where c 1 and c 2 are taken as averages over the reaction section under study, corresponding to Δt.
6. Ostwald isolation method.
Let's write the kinetic equation of the reaction: u = kс A n 1 ´с B n 2 ´с With n 3.
We increase the concentration of “B” and “C” by more than 10 times. The order for these substances will be zero, their concentrations will not change. We determine “n 1” using one of the methods discussed above. We do the same when determining the order of the reaction based on substances B and C, i.e. n 2 and n 3.
1. Basic concepts and postulates of chemical kinetics
Chemical kinetics is a branch of physical chemistry that studies the rates of chemical reactions. The main tasks of chemical kinetics: 1) calculation of reaction rates and determination of kinetic curves, i.e. dependence of the concentrations of reactants on time ( direct task); 2) determination of reaction mechanisms from kinetic curves ( inverse problem).
The rate of a chemical reaction describes the change in concentrations of reactants per unit time. For reaction
a A+ b B+... d D+ e E+...
the reaction rate is determined as follows:
where square brackets indicate the concentration of the substance (usually measured in mol/l), t- time; a, b, d, e- stoichiometric coefficients in the reaction equation.
The reaction rate depends on the nature of the reactants, their concentration, temperature and the presence of a catalyst. The dependence of the reaction rate on concentration is described by the basic postulate of chemical kinetics - law of mass action:
The rate of a chemical reaction at each moment in time is proportional to the current concentrations of the reactants, raised to certain powers:
![]() ,
,
Where k- rate constant (independent of concentration); x, y- some numbers that are called order of reaction by substance A and B, respectively. In general, these numbers have nothing to do with the coefficients a And b in the reaction equation. Sum of exponents x+ y called general reaction order. The order of the reaction can be positive or negative, integer or fractional.
Most chemical reactions consist of several steps called elementary reactions. An elementary reaction is usually understood as a single act of formation or cleavage of a chemical bond, proceeding through the formation of a transition complex. The number of particles participating in an elementary reaction is called molecularity reactions. There are only three types of elementary reactions: monomolecular (A B + ...), bimolecular (A + B D + ...) and trimolecular (2A + B D + ...). For elementary reactions, the overall order is equal to the molecularity, and the orders by substance are equal to the coefficients in the reaction equation.
EXAMPLES
Example 1-1. The rate of NO formation in the reaction 2NOBr (g) 2NO (g) + Br 2 (g) is 1.6. 10 -4 mol/(l.s). What is the rate of reaction and the rate of NOBr consumption?
Solution. By definition, the reaction rate is:
Mol/(l.s).
From the same definition it follows that the rate of NOBr consumption is equal to the rate of NO formation with the opposite sign:
![]() mol/(l.s).
mol/(l.s).
Example 1-2. In the 2nd order reaction A + B D, the initial concentrations of substances A and B are equal to 2.0 mol/L and 3.0 mol/L, respectively. The reaction rate is 1.2. 10 -3 mol/(l.s) at [A] = 1.5 mol/l. Calculate the rate constant and reaction rate at [B] = 1.5 mol/L.
Solution. According to the law of mass action, at any moment of time the reaction rate is equal to:
![]() .
.
By the time when [A] = 1.5 mol/l, 0.5 mol/l of substances A and B have reacted, so [B] = 3 – 0.5 = 2.5 mol/l. The rate constant is:
L/(mol. s).
By the time when [B] = 1.5 mol/l, 1.5 mol/l of substances A and B have reacted, therefore [A] = 2 – 1.5 = 0.5 mol/l. The reaction rate is:
Mol/(l.s).
TASKS
1-1. How is the rate of the ammonia synthesis reaction 1/2 N 2 + 3/2 H 2 = NH 3 expressed in terms of the concentrations of nitrogen and hydrogen? (answer)
1-2. How will the rate of the ammonia synthesis reaction 1/2 N 2 + 3/2 H 2 = NH 3 change if the reaction equation is written as N 2 + 3H 2 = 2NH 3? (answer)
1-3. What is the order of elementary reactions: a) Cl + H 2 = HCl + H; b) 2NO + Cl 2 = 2NOCl? (answer)
1-4. Which of the following quantities can take a) negative; b) fractional values: reaction rate, reaction order, reaction molecularity, rate constant, stoichiometric coefficient? (answer)
1-5. Does the rate of a reaction depend on the concentration of reaction products? (answer)
1-6. How many times will the rate of the gas-phase elementary reaction A = 2D increase when the pressure increases by 3 times? (answer)
1-7. Determine the order of the reaction if the rate constant has the dimension l 2 / (mol 2 . s). (answer)
1-8. The rate constant of a 2nd order gas reaction at 25 o C is equal to 10 3 l/(mol. s). What is this constant equal to if the kinetic equation is expressed in terms of pressure in atmospheres? (answer)
1-9. For gas phase reaction n th order nA B, express the rate of formation of B in terms of the total pressure. (answer)
1-10. The rate constants for the forward and reverse reactions are 2.2 and 3.8 l/(mol. s). By which of the following mechanisms can these reactions occur: a) A + B = D; b) A + B = 2D; c) A = B + D; d) 2A = B.(answer)
1-11. The decomposition reaction 2HI H 2 + I 2 has a 2nd order with a rate constant k= 5.95. 10 -6 l/(mol. s). Calculate the reaction rate at a pressure of 1 atm and a temperature of 600 K. (answer)
1-12. The rate of the 2nd order reaction A + B D is 2.7. 10 -7 mol/(l.s) at concentrations of substances A and B, respectively, 3.0. 10 -3 mol/l and 2.0 mol/l. Calculate the rate constant.(answer)
1-13. In the 2nd order reaction A + B 2D, the initial concentrations of substances A and B are equal to 1.5 mol/l. The reaction rate is 2.0. 10 -4 mol/(l.s) at [A] = 1.0 mol/l. Calculate the rate constant and reaction rate at [B] = 0.2 mol/L. (answer)
1-14. In the 2nd order reaction A + B 2D, the initial concentrations of substances A and B are equal to 0.5 and 2.5 mol/l, respectively. How many times is the reaction rate at [A] = 0.1 mol/l less than the initial rate? (answer)
1-15. The rate of the gas-phase reaction is described by the equation w = k. [A] 2 . [B]. At what ratio between the concentrations of A and B will the initial reaction rate be maximum at a fixed total pressure? (answer)
2. Kinetics of simple reactions
In this section, based on the law of mass action, we will compose and solve kinetic equations for irreversible reactions of a whole order.
0th order reactions. The rate of these reactions does not depend on concentration:
![]() ,
,
where [A] is the concentration of the starting substance. Zero order occurs in heterogeneous and photochemical reactions.
1st order reactions. In type A–B reactions, the rate is directly proportional to the concentration:
![]() .
.
When solving kinetic equations, the following notation is often used: initial concentration [A] 0 = a, current concentration [A] = a - x(t), Where x(t) is the concentration of the reacted substance A. In this notation, the kinetic equation for the 1st order reaction and its solution have the form:
The solution to the kinetic equation is also written in another form, convenient for analyzing the reaction order:
![]() .
.
The time during which half of substance A decays is called the half-life t 1/2. It is defined by the equation x(t 1/2) = a/2 and equal
2nd order reactions. In reactions of type A + B D + ..., the rate is directly proportional to the product of concentrations:
![]() .
.
Initial concentrations of substances: [A] 0 = a, [B] 0 = b; current concentrations: [A] = a- x(t), [B] = b - x(t).
When solving this equation, two cases are distinguished.
1) identical initial concentrations of substances A and B: a = b. The kinetic equation has the form:
![]() .
.
The solution to this equation is written in various forms:
The half-lives of substances A and B are the same and equal to:
2) The initial concentrations of substances A and B are different: a
b. The kinetic equation has the form: ![]() .
.
The solution to this equation can be written as follows:
The half-lives of substances A and B are different: ![]() .
.
Nth order reactions n A D + ... The kinetic equation has the form:
![]() .
.
Solution of the kinetic equation:
 . (2.1)
. (2.1)
The half-life of substance A is inversely proportional to ( n-1)th degree of initial concentration:
![]() . (2.2)
. (2.2)
Example 2-1. The half-life of the radioactive isotope 14 C is 5730 years. During archaeological excavations, a tree was found whose 14 C content was 72% of normal. How old is the tree?
Solution. Radioactive decay is a 1st order reaction. The rate constant is:
The life time of a tree can be found from solving the kinetic equation, taking into account the fact that [A] = 0.72. [A] 0:
Example 2-2. It has been established that a 2nd order reaction (one reagent) is 75% complete in 92 minutes at an initial reagent concentration of 0.24 M. How long will it take for the reagent concentration to reach 0.16 M under the same conditions?
Solution. Let us write the solution of the kinetic equation for a 2nd order reaction with one reagent twice:
![]() ,
,
where, by condition, a= 0.24 M, t 1 = 92 min, x 1 = 0.75. 0.24 = 0.18 M, x 2 = 0.24 - 0.16 = 0.08 M. Let's divide one equation by another:
Example 2-3. For an elementary reaction n A B we denote the half-life of A by t 1/2, and the decay time of A by 75% by t 3/4. Prove that the ratio t 3/4 / t 1/2 does not depend on the initial concentration, but is determined only by the order of the reaction n.Solution. Let us write the solution of the kinetic equation for the reaction twice n-th order with one reagent:
and divide one expression by another. Constants k And a both expressions will cancel and we get:
![]() .
.
This result can be generalized by proving that the ratio of the times for which the degree of conversion is a and b depends only on the order of the reaction:
 .
.
TASKS
2-1. Using the solution to the kinetic equation, prove that for 1st order reactions the time t x, during which the degree of conversion of the starting substance reaches x, does not depend on the initial concentration. (answer)2-2. The first order reaction proceeds 30% in 7 minutes. How long will it take for the reaction to be 99% complete? (answer)
2-3. The half-life of the radioactive isotope 137 Cs, which entered the atmosphere as a result of the Chernobyl accident, is 29.7 years. After what time will the amount of this isotope be less than 1% of the original? (answer)
2-4. The half-life of the radioactive isotope 90 Sr, which enters the atmosphere during nuclear tests, is 28.1 years. Let's assume that the body of a newborn child absorbed 1.00 mg of this isotope. How much strontium will remain in the body after a) 18 years, b) 70 years, if we assume that it is not excreted from the body? (answer)
2-5. The rate constant for the first order reaction SO 2 Cl 2 = SO 2 + Cl 2 is 2.2. 10 -5 s -1 at 320 o C. What percentage of SO 2 Cl 2 will decompose when kept for 2 hours at this temperature? (answer)
2-6. 1st order reaction rate constant
2N 2 O 5 (g) 4NO 2 (g) + O 2 (g)
at 25 o C is equal to 3.38. 10 -5 s -1 . What is the half-life of N 2 O 5? What will be the pressure in the system after a) 10 s, b) 10 min, if the initial pressure was 500 mm Hg? Art. (answer)
2-7. The first order reaction is carried out with varying amounts of the starting material. Will the tangents to the initial sections of the kinetic curves intersect at one point on the x-axis? Explain your answer. (answer)
2-8. The first order reaction A 2B occurs in the gas phase. The initial pressure is p 0 (B missing). Find the dependence of total pressure on time. After what time will the pressure increase by 1.5 times compared to the original? What is the progress of the reaction by this time? (answer)
2-9. The second order reaction 2A B occurs in the gas phase. The initial pressure is p 0 (B missing). Find the dependence of total pressure on time. After what time will the pressure decrease by 1.5 times compared to the original? What is the progress of the reaction by this time? (answer)
2-10. Substance A was mixed with substances B and C in equal concentrations of 1 mol/l. After 1000 s, 50% of substance A remains. How much substance A will remain after 2000 s if the reaction has: a) zero, b) first, c) second, c) third general order? (answer)
2-11. Which of the reactions - first, second or third order - will end faster if the initial concentrations of substances are 1 mol/l and all rate constants expressed in terms of mol/l and s are equal to 1? (answer)
2-12. Reaction
CH 3 CH 2 NO 2 + OH - H 2 O + CH 3 CHNO 2 -
has second order and rate constant k= 39.1 l/(mol. min) at 0 o C. A solution was prepared containing 0.004 M nitroethane and 0.005 M NaOH. How long will it take for 90% of nitroethane to react?
2-13. The rate constant for the recombination of H + and FG - (phenylglyoxynate) ions into an UFG molecule at 298 K is equal to k= 10 11.59 l/(mol. s). Calculate the time it takes for the reaction to complete 99.999% if the initial concentrations of both ions are 0.001 mol/L. (answer)
2-14. The rate of oxidation of 1-butanol by hypochlorous acid does not depend on the alcohol concentration and is proportional to 2. How long will it take for the oxidation reaction at 298 K to complete 90% if the initial solution contained 0.1 mol/L HClO and 1 mol/L alcohol? The reaction rate constant is k= 24 l/(mol min). (answer)
2-15. At a certain temperature, a 0.01 M ethyl acetate solution is saponified by a 0.002 M NaOH solution by 10% in 23 minutes. After how many minutes will it be saponified to the same degree with a 0.005 M KOH solution? Consider that this reaction is of second order, and the alkalis are completely dissociated. (answer)
2-16. The second order reaction A + B P is carried out in a solution with initial concentrations [A] 0 = 0.050 mol/L and [B] 0 = 0.080 mol/L. After 1 hour, the concentration of substance A decreased to 0.020 mol/l. Calculate the rate constant and half-lives of both substances.
To experimentally determine the rate of a chemical reaction, it is necessary to have data on changes in the concentration of initial or final substances over time. The methods by which this can be done are divided into chemical And physico-chemical.
Chemical methods are based on the direct determination of the amount of a substance or its concentration in a reaction vessel.
Most often, such types of quantitative analysis as titrimetry and gravimetry are used for these purposes. If the reaction proceeds slowly, then to monitor the consumption of reagents, samples are taken from the reaction medium at certain intervals. Then the content of the desired substance is determined. For example, titration with alkali determines the amount of acid in the system as the reaction proceeds
R 1 – COOH + R 2 – OH → R 1 – COO – R 2 + H 2 O
If the reaction proceeds at high speed, then to take a sample it is stopped by sudden cooling, rapid removal of the catalyst, dilution, or transfer of one of the reagents to a non-reactive state.
Chemical methods of analysis are characterized by simplicity, accessibility and good accuracy.
In modern experimental kinetics, they most often use physico-chemical methods of analysis . They allow you to control changes in the concentration of a substance directly during the reaction, without stopping it or taking a sample. These methods are based on measuring any physical property of a system that changes over time and depends on the quantitative content of a certain compound in it; for example: pressure (if gases are involved in the reaction), electrical conductivity, refractive index, absorption spectrum of the reagent or reaction product in the ultraviolet, visible or infrared regions. Electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR) spectra are widely used.
The use of spectral methods is based on the fact that the absorption of electromagnetic radiation is proportional to the amount of a substance or its concentration in the system.
Usually reactions are studied experimentally in a closed system (i.e. at constant volume) and the results are presented graphically in the form of the so-called kinetic curve, expressing the dependence of the concentration of the reagent or reaction product on timet. The analytical form of this dependence is called kinetic curve equation. In contrast to the basic kinetic equation, the equations for the consumption curves of reacting substances (or accumulation of reaction products) contain as parameters the initial concentrations of the components (C 0) at time t = 0.
From these equations, formulas are derived to calculate the reaction rate constant and half-life time(t½) – period of time during which half of the taken starting substance is consumed, i.e. its concentration will decrease by 2 times and become equal to C O /2.
In zero-order reactions, the concentration of the starting substance decreases linearly with time (Fig. 37)
Rice. 37. Change in the concentration of the starting substance over time in a zero-order reaction
Mathematically, this linear relationship can be written as follows:
Wherek– rate constant, C 0 – initial molar concentration of the reagent, C – concentration at timet.
From it we can derive a formula for calculating the rate constant of a zero-order chemical reaction.
k = (C 0 – C).
The zero order rate constant is measured in mol/l ∙ s (mol l -1 · With -1 ).
The half-conversion time for a zero-order reaction is proportional to the concentration of the starting material
For first-order reactions, the kinetic curve in coordinates C, t is exponential in nature and looks like this (Fig. 38) Mathematically, this curve is described by the following equation
C = C 0 e - kt

Rice. 38. Change in the concentration of the starting substance over time in a first-order reaction
In practice, for first-order reactions, the kinetic curve is most often plotted in coordinates ℓnC,t. In this case, a linear dependence of ℓnС on time is observed (Fig. 39)
ℓnС = ℓnС 0 –kt

Rice. 39. Dependence of the logarithm of the reagent concentration on the time of occurrence for a first-order reaction
Accordingly, the value of the rate constant and half-conversion time can be calculated using the following formulas
k = ℓn or k= 2.303ℓg
(when moving from the decimal logarithm to the natural one).
The first order reaction rate constant has the dimension t –1 , i.e. 1/s and does not depend on the concentration units. It shows the proportion of molecules that react per unit time of the total number of reagent molecules in the system. Thus, in first-order reactions, equal fractions of the taken amount of the starting substance are consumed over equal periods of time.
The second distinctive feature of first-order reactions is that t ½ for them does not depend on the initial concentration of the reagent, but is determined only by the rate constant.
We will consider the form of the equation for the dependence of concentration on time for second-order reactions only for the simplest case, when 2 identical molecules or molecules of different substances participate in an elementary act, but their initial concentrations (C 0) are equal. In this case, a linear dependence is observed in coordinates 1/C,t (Fig. 40). The mathematical equation for this relationship will be written as follows:

Rice. 40. Dependence of the inverse concentration of the reagent on time for a second-order reaction
Constant speed is calculated by the formula
and is measured in hp -1 ∙mol -1 , i.e. its numerical value depends on the units in which the concentration of the substance is measured.
The half-life of second-order reactions is inversely proportional to the initial concentration of the reagent
This is due to the fact that the rate of second-order reactions strongly depends on the number of collisions between molecules of reacting substances per unit time, which, in turn, is proportional to the number of molecules per unit volume, i.e. concentration of the substance. Thus, the greater the concentration of a substance in the system, the more often the molecules collide with each other and the less time half of them will have time to react.
Third-order reactions, as mentioned earlier, are extremely rare and are of no practical interest. Therefore, in this regard, we will not consider them.
reaction rate constant- is the rate of a chemical reaction under conditions when the product of the concentrations of the reacting substances is 1 mol/l. General chemistry: textbook / A. V. Zholnin Reaction rate constant - proportionality coefficient in differential kinetic... ... Chemical terms
reaction rate constant- - [A.S. Goldberg. English-Russian energy dictionary. 2006] Topics: energy in general EN reaction constant ...
reaction rate constant- reakcijos greičio konstanta statusas T sritis chemija apibrėžtis Reakcijos, kurios reaguojančiųjų medžiagų koncentracijos lygios vienetui, greitis. atitikmenys: engl. rate constant; reaction constant rus. reaction rate constant; specific... ... Chemijos terminų aiškinamasis žodynas
reaction rate constant- reakcijos spartos konstanta statusas T sritis Standartizacija ir metrologija apibrėžtis Reakcijos, kurios reaguojančių medžiagų koncentracijos yra lygios vienetui, sparta. atitikmenys: engl. reaction rate constant vok. Reaktionskonstante, f rus.… … Penkiakalbis aiškinamasis metrologijos terminų žodynas
A chemical reaction is its main kinetic characteristic; coefficient of proportionality in the kinetic equation relating the reaction rate to the concentrations of reactants and their stoichiometric coefficients. For monomolecular... ... Big Encyclopedic Dictionary
catalytic reaction rate constant- - [A.S. Goldberg. English-Russian energy dictionary. 2006] Energy topics in general EN catalytic coefficient ... Technical Translator's Guide
Chemical reaction, its main kinetic characteristics; coefficient of proportionality in the kinetic equation relating the reaction rate to the concentrations of reactants and their stoichiometric coefficients. For monomolecular... ... encyclopedic Dictionary
chemical reaction rate constant- change in the amount (concentration) of a substance that reacts or is formed during the process per unit time at a given temperature and concentrations of all components equal to unity: d[A]/dt =… … Encyclopedic Dictionary of Metallurgy
Chem. reaction, its main kinetic. characteristic; coefficient proportionality in kinetic. equation connecting the reaction rate with the concentrations of reactants in and their stoichiometric. coefficients. For monomolecular reactions K. s. has a dimension with... Natural science. encyclopedic Dictionary
Relative rate constants for the reaction CH 3 I + Cl - in different solvents at 25 °C (according to Parker)- Solvent Relative rate constant CH3OH 1 HCONH2 12.5 HCONHCH3 … Chemical reference book
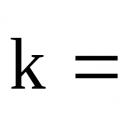 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe