Electricity: general concepts. A molecule of a substance is the smallest particle that preserves its properties. What is the smallest particle of a substance that preserves its properties?
Molecular structure of matter. Speeds of gas molecules.
The molecular kinetic theory of MKT is a theory that explains the properties of a substance based on its molecular structure. The main provisions of the molecular kinetic theory: all bodies consist of molecules; molecules are constantly moving; molecules interact with each other.
Molecule– the smallest particle of a substance that retains the properties of a given substance.
Atoms– the smallest particle of a chemical element. Molecules are made up of atoms.
Molecules are constantly moving. The proof of this position is diffusion- the phenomenon of penetration of molecules of one substance into another. Diffusion occurs in gases, liquids, and solids. As temperature increases, the rate of diffusion increases. The movement of paint particles in a solution discovered by Brown is called Brownian motion and also proves the movement of molecules.
Atomic structure. An atom consists of a positively charged nucleus around which electrons orbit.
Atomic nucleus consists of nucleons (proton, neutron). The charge of the nucleus is determined by the number of protons. The mass number is determined by the number of nucleons. Isotopes are atoms of the same element whose nuclei contain different numbers of neutrons.
Relative atomic mass M – mass of one atom in units atomic mass (1/12 the mass of a carbon atom). Relative molecular weight– M is the mass of the molecule in atomic mass units.
Quantity of substance determined by the number of molecules. A mole is a unit of measurement for the amount of a substance. Mole- the amount of a substance whose mass, expressed in grams, is numerically equal to the relative molecular mass. 1 mole substance contains N A molecules. N A = 6,022∙10 23 1/mol – Avogadro’s number. The mass of one mole in kilograms is called molar mass – μ =M·10 -3 . 1 mol – 12gC – N A -22.4 l. gas
Number moles is determined by the formulas : ν = m / μ , ν = N / N A , ν = V / V 0 .
Basic MKT model– a set of moving and interacting molecules of a substance. Aggregate states of matter.
Solid: W P >> W k, the packing is dense, the molecules vibrate around the equilibrium position, the equilibrium positions are stationary, the arrangement of the molecules is ordered, i.e. a crystal lattice is formed, and both shape and volume are preserved.
Liquid:W P ≈ W k , the packing is dense, the molecules vibrate around the equilibrium position, the equilibrium positions are mobile, the arrangement of molecules is ordered within 2, 3 layers (short-range order), the volume is preserved, but the shape is not preserved (fluidity).
Gas: W P W k , molecules are located far from each other, move rectilinearly until they collide with each other, the collisions are elastic, they easily change both shape and volume. Ideal gas conditions: W P =0, collisions are perfectly elastic, Diameter of molecule distances between them.
Plasma – electrically neutral collection of neutral and charged particles . Plasma(gas) molecules are located far from each other, move rectilinearly until they collide with each other, easily change both shape and volume, collisions are inelastic, ionization occurs during collisions, and reacts to electric and magnetic fields.
Phase transitions: evaporation, condensation, sublimation, melting, crystallization.
Statistical patterns– laws of behavior of a large number of particles. Microparameters– small-scale parameters – mass, size, speed and other characteristics of molecules and atoms. Macro parameters – parameters of large scales - mass, volume, pressure, temperature of physical bodies.
R
Z =2 N
distribution of ideal gas particles over two halves of a vessel:
Number of possible statesZwith the number of particlesN is found by the formula
H
Z = N! / n!∙(N-n)!
number of ways to implement staten/ (N – n) is found by the formula
Analysis of the answers leads to the conclusion that there is the greatest probability that the molecules will be distributed equally among the two halves of the vessels.
The most probable speed is the speed that most molecules have
How to calculate the average speed of molecules V av = (V 1 ∙ N 1 + V 2 ∙ N 2 + V 3 ∙ N 3)/N. The average speed is usually higher than the most likely speed.
Communication: speed – energy – temperature. E cf ~ T.
T
E=3 kT /2
temperature determines the degree of body heating. Temperature main characteristic bodies in thermal equilibrium. Thermal equilibrium when there is no heat exchange between bodies
Temperature is a measure of the average kinetic energy of gas molecules. With increasing temperature, the rate of diffusion increases and the speed of Brownian motion increases. The formula for the relationship between the average kinetic energy of molecules and temperature is expressed by the formula gdk k = 1.38∙10 -23 J/K – Boltzmann’s constant, expressing the relationship between Kelvin and Joule as units of temperature.
T
T = t + 273.
thermodynamic temperature cannot be negative.
Absolute temperature scale– Kelvin scale (273K – 373K).
Temperature scales: Celsius (0 o C – 100 o C), Fahrenheit (32 o F – 212 o F), Kelvin (273K – 373K).
Speed of thermal movement of molecules: m 0 v 2 = 3 kT, v 2 = 3 kT / m 0 , v 2 = 3 kN A T / μ

Gas laws
Pressure is a macroscopic parameter of the system . Pressure is numerically equal to the force acting per unit surface perpendicular to this surface.P= F/ S. Pressure is measured in Pascals (Pa), atmospheres (atm.), bars (bar), mmHg. The pressure of a column of gas or liquid in a gravitational field is found by the formula P = ρgh, where ρ is the density of the gas or liquid, h is the height of the column. In communicating vessels, a homogeneous liquid is established at the same level. The ratio of the heights of columns of inhomogeneous liquids is inverse to the ratio of their densities.
Atmosphere pressure– pressure created by the Earth’s air shell. Normal atmospheric pressure is 760 mm Hg. or 1.01∙10 5 Pa, or 1 bar, or 1 atm.
Gas pressure is determined the number of molecules hitting the wall of the container and their speed.
Arithmetic average speed the movement of gas molecules is zero, because there is no advantage to movement in any particular direction due to the fact that the movement of molecules is equally probable in all directions. Therefore, to characterize the movement of molecules we take root mean square speed. Average squares of speed axes X,Y,Z are equal to each other and amount to 1/3 of the root mean square speed.


 For one mole of gas
For one mole of gas




Isobars
P 1
Gay-Lussac's law

V = const – isochoric process,

Isochores
V 1
Charles's law.
Tasks: Task № 1 . Define full number microstates of six particles of an ideal gas in two halves of a vessel not separated by a partition. What is the number of ways to realize states 1/5, 2/4? At what state will the number of implementation methods be maximum?
Solution. Z =2 N = 2 6 = 64. For state 1/5 Z = N! / n!∙(N-n)! = 1∙2∙3∙4∙5∙6 / 1∙1∙2∙3∙4∙5= 6
On one's own. What is the number of ways to implement states 2/4?
Task No. 2. Find the number of molecules in a glass of water (m=200g). Solution. N = m∙ N A /μ = 0.2 ∙ 6.022∙10 23 / 18 ∙ 10 -3 =67∙ 10 23 .
On one's own. Find the number of molecules in 2 g of copper. Find the number of molecules in 1 m 3 of carbon dioxide CO 2 .
Task No. 3. The figure shows a closed loop in coordinates P V. What processes occurred with the gas? How did macro parameters change? Draw this diagram in VT coordinates.
WITH  independently draw the diagram in PT coordinates.
independently draw the diagram in PT coordinates.
|
P |
V |
T |
|
|
1-2 |
uv |
fast |
uv |
|
2-3 |
fast |
uv |
uv |
|
3-4 |
mind |
uv |
fast |
|
4-1 |
fast |
mind |
mind |
 decision.
decision.
Task No. 4."Magdeburg Hemispheres" stretched 8 horses on each side. How will the traction force change if one hemisphere is attached to a wall and the other is pulled by 16 horses?
Z  task number 5. An ideal gas exerts a pressure of 1.01∙10 5 Pa on the walls of the vessel. The thermal speed of molecules is 500 m/s. Find the gas density. (1.21kg/m3). Solution.. Let's divide both sides of the equation by V. We get
task number 5. An ideal gas exerts a pressure of 1.01∙10 5 Pa on the walls of the vessel. The thermal speed of molecules is 500 m/s. Find the gas density. (1.21kg/m3). Solution.. Let's divide both sides of the equation by V. We get


μ we find from the formula for the speed of molecules
Task No. 6. What pressure is oxygen under if the thermal speed of its molecules is 550 m/s, and their concentration 10 25 m -3 ? (54kPa.) Solution. P = nkT, R=N A k,P=nv 2 μ /3N A , We find T from the formula
Task No. 7. Nitrogen occupies a volume of 1 liter at normal atmospheric pressure. Determine the energy of translational motion of gas molecules.
Solution. Energy of one molecule - E o = 5 kT / 2 , energy of all molecules in a given volume of gas – E = N 5 kT / 2 = nV 5 kT / 2, P = nkT , E = 5 PV /2 = 250 J.
Task № 8. Air consists of a mixture of nitrogen, oxygen and argon. Their concentrations are respectively 7.8 ∙ 10 24 m -3 , 2.1 ∙ 10 24 m -3 , 10 23 m -3 . Average kinetic energy molecules of the mixture is the same and equal to 3 ∙10 -21 J. Find the air pressure. (20kPa). On one's own.
Task No. 9. How will the gas pressure change when its volume decreases by 4 times and the temperature increases by 1.5 times? (Increases 6 times). On one's own.
Task No. 10. The gas pressure in a fluorescent lamp is 10 3 Pa, and its temperature is 42 o C. Determine the concentration of atoms in the lamp. Estimate the average distance between molecules.
(2.3∙10 23 m -3, 16.3 nm). On one's own.
Task No. 11. Find the volume of one mole of an ideal gas of any chemical composition under normal conditions. (22.4l). On one's own.
Z  problem number 12. A vessel with a volume of 4 liters contains molecular hydrogen and helium. Assuming the gases are ideal, find the pressure of the gases in the vessel at a temperature of 20 o C if their masses are 2g and 4g, respectively. (1226kPa).
problem number 12. A vessel with a volume of 4 liters contains molecular hydrogen and helium. Assuming the gases are ideal, find the pressure of the gases in the vessel at a temperature of 20 o C if their masses are 2g and 4g, respectively. (1226kPa).
Solution. According to Dalton's law P = P 1 + R 2 . We find the partial pressure of each gas using the formula. Both hydrogen and helium occupy the entire volume V=4l.
Problem No. 13. Determine the depth of the lake if the volume of the air bubble doubles as it rises from the bottom to the surface. The temperature of the bubble does not have time to change. (10.3m).
Solution. The process is isothermal P 1 V 1 = P 2 V 2
The pressure in a bubble on the surface of the water is equal to atmospheric pressure P 2 = P o The pressure at the bottom of the reservoir is the sum of the pressure inside the bubble and the pressure of the water column R 1 = P O + ρ gh, where ρ = 1000 kg/m 3 is the density of water, h is the depth of the reservoir. R O = (R O + ρ gh) V 1 / 2 V 1 = (R O + ρ gh)/ 2
Problem No. 14. The cylinder is divided by an impenetrable fixed partition into two parts, the volumes of which are V 1, V 2. The air pressure in these parts of the cylinder is P 1, P 2, respectively. When the fastening is removed, the partition can move like a weightless piston. How much and in which direction will the partition move?
R
P 1 V 1
P 2 V 2
decision . If P 2 > P 1 Pressure in both parts
P 1 V 1 = P (V 1 -∆ V)
P 2 V 2 = P (V 2 + ∆ V)
the cylinder will be set to the same - R. The process is isothermal.
Let's divide the right and left sides of the equations into each other. And then we solve the equation for ∆ V.
Answer: ((P 1 – P 2 ) V 1 V 2 )/(P 1 V 1 + P 2 V 2 .
Problem No. 15. Car tires are inflated to a pressure of 2∙10 4 Pa at a temperature of 7 o C. A few hours after driving, the air temperature in the tires rose to 42 o C. What was the pressure in the tires? (2.25∙10 4 Pa). On one's own.

Theory of the structure of matter
Complete the sentences
The smallest particle of a substance that retains its properties - molecule
Molecules consist from atoms
The molecules of the same substance are the same
Different substances have molecules different
When a substance is heated, the size of the molecules do not change

“A sea drop by a drop, a haystack by a blade of grass”
What position of the theory of the structure of matter is this proverb talking about?

“When I go into the water, I’m red, when I come out, I’m black.”
How do the distances between particles of a substance change?

Diffusion Diffusio (lat.) – distribution, spreading
The phenomenon of spontaneous penetration of substances into each other

Diffusion in gases

Diffusion in liquids

Diffusion in solids

Reason for diffusion

The intensity of diffusion depends on the state of the substance

Diffusion intensity depends on temperature

Brownian motion
the movement of very small particles of a substance visible through a microscope under the influence of molecular impacts.

Model of "Brownian motion"

Conclusion
The smell of grass or the smell of perfume
Aroma of forest berries and flowers
I can only explain it by diffusion
I understand this phenomenon.
The essence is all in the movement of particles of matter
Everything is as clear to me as two and two.

A little poetry... A beautiful lady was smelling roses. And she sneezed and tears began to fall.
Is it really due to diffusion?
Do such confusions exist?
Explain the saying
A fly in the ointment will spoil a barrel of honey.

A little history...
The English metallurgist William Roberts-Austin measured the diffusion of gold in lead. He fused a thin disk of gold onto the end of a 1 inch (2.45 cm) long cylinder of pure lead, placed the cylinder in a furnace where the temperature was maintained at about 200°C, and kept it in the furnace for 10 days. He then cut the cylinder into thin disks. It turned out that by the “clean” end a quite measurable amount of gold had passed through the entire lead cylinder.

Diffusion in the kitchen
Cucumbers or tomatoes Pickling is no problem. Boil the brine, throw in the salt, and are ready for lunch.

The smallest particle of a chemical element that can exist independently is called an atom.
An atom is the smallest particle of a chemical element, indivisible only in chemical terms.
An atom is the smallest particle of a chemical element that retains everything Chemical properties this element. Atoms can exist in a free state and in compounds with atoms of the same or other elements.
An atom is the smallest particle of a chemical element that can exist independently.
According to modern views, an atom is the smallest particle of a chemical element, possessing all its chemical properties. By connecting with each other, atoms form molecules, which are the smallest particles of a substance - carriers of all its chemical properties.
The previous chapter outlined our ideas about. atom - the smallest particle of a chemical element. The smallest particle of a substance is a molecule formed from atoms between which chemical forces act, or chemical bond.
The concept of electricity is inextricably linked with the concept of the structure of atoms - the smallest particles of a chemical element.
From chemistry and previous sections of physics, we know that all bodies are built from individual, very small particles - atoms and molecules. By atoms we mean the smallest particle of a chemical element. A molecule is a more complex particle consisting of several atoms. The physical and chemical properties of elements are determined by the properties of the atoms of these elements.
Decisive in the establishment of atomistic concepts in chemistry were the works of the English scientist John Dalton (1766 - 1844), who introduced into chemistry the term atom itself as the smallest particle of a chemical element; atoms of different elements, according to Dalton, have different masses and thus differ from each other.
An atom is the smallest particle of a chemical element, a complex system consisting of a central positively charged nucleus and a shell of negatively charged particles moving around the nucleus - electrons.
From chemistry and previous sections of physics we know that all bodies are built from individual, very small particles - atoms and molecules. Atoms are the smallest particles of a chemical element. A molecule is a more complex particle consisting of several atoms. The physical and chemical properties of elements are determined by the properties of the atoms of these elements.
From chemistry and previous sections of physics we know that all bodies are built from individual, very small particles - atoms and molecules. An atom is the smallest particle of a chemical element. A molecule is a more complex particle consisting of several atoms. The physical and chemical properties of elements are determined by the properties of the atoms of these elements.
Phenomena confirming the complex structure of the atom. The structure of an atom - the smallest particle of a chemical element - can be judged, on the one hand, by the signals that it itself sends in the form of rays and even particles, on the other, by the results of bombardment of atoms of matter by fast charged particles.
The idea that all bodies consist of extremely small and further indivisible particles - atoms - was widely discussed even before our era by ancient Greek philosophers. Modern idea of atoms as the smallest particles chemical elements, capable of binding into larger particles - molecules that make up substances, was first expressed by M. V. Lomonosov in 1741 in his work Elements of Mathematical Chemistry; These views were propagated by him throughout his entire career. scientific activity. Contemporaries did not pay due attention to the works of M.V. Lomonosov, although they were published in publications of the St. Petersburg Academy of Sciences, received by all major libraries of that time.
The idea that all bodies consist of extremely small and further indivisible particles - atoms - was discussed back in Ancient Greece. The modern idea of atoms as the smallest particles of chemical elements capable of bonding into larger particles - molecules that make up substances, was first expressed by M. V. Lomonosov in 1741 in his work Elements of Mathematical Chemistry; He propagated these views throughout his entire scientific career.
The idea that all bodies consist of extremely small and further indivisible particles - atoms - was widely discussed even before our era by ancient Greek philosophers. The modern idea of atoms as the smallest particles of chemical elements capable of bonding into larger particles - molecules that make up substances, was first expressed by M. V. Lomonosov in 1741 in his work Elements of Mathematical Chemistry; He propagated these views throughout his entire scientific career.
The idea that all bodies consist of extremely small and further indivisible particles - atoms - was widely discussed by ancient Greek philosophers. The modern idea of atoms as the smallest particles of chemical elements capable of bonding into larger particles - molecules that make up substances, was first expressed by M. V. Lomonosov in 1741 in his work Elements of Mathematical Chemistry; He propagated these views throughout his entire scientific career.
All kinds of quantitative calculations of the masses and volumes of substances participating in chemical reactions. In this regard, stoichiometric laws quite rightly relate to the basic laws of chemistry and are a reflection of the real existence of atoms and molecules that have a certain mass of the smallest particles of chemical elements and their compounds. Because of this, the stoichiometric laws became a solid foundation, on which modern atomic-molecular science was built.
All kinds of quantitative calculations of the masses and volumes of substances taking part in chemical reactions are based on stoichiometric laws. In this regard, stoichiometric laws quite rightly relate to the basic laws of chemistry and are a reflection of the real existence of atoms and molecules that have a certain mass of the smallest particles of chemical elements and their compounds. Because of this, stoichiometric laws became a solid foundation on which modern atomic-molecular science was built.
Phenomena confirming the complex structure of the atom. The structure of an atom - the smallest particle of a chemical element - can be judged, on the one hand, by the signals it sends in the form of rays and even particles, and on the other hand, by the results of bombardment of atoms of matter by fast charged particles.
It should be noted that the creation quantum physics was directly stimulated by attempts to understand the structure of the atom and the patterns of emission spectra of atoms. As a result of experiments, it was discovered that at the center of the atom there is a small (compared to its size) but massive nucleus. An atom is the smallest particle of a chemical element that retains its properties. It gets its name from the Greek dtomos, which means indivisible. The indivisibility of the atom occurs in chemical transformations, as well as during collisions of atoms occurring in gases. And at the same time, the question has always arisen whether the atom consists of smaller parts.
The object of study in chemistry is chemical elements and their compounds. Chemical elements are collections of atoms with identical nuclear charges. In turn, an atom is the smallest particle of a chemical element that retains all its chemical properties.
The essence of this rejection of Avogadro's hypothesis was the reluctance to introduce a special concept of a molecule (particle), reflecting a discrete form of matter qualitatively different from atoms. Indeed: Dalton's simple atoms correspond to the smallest particles of chemical elements, and his complex atoms correspond to the smallest particles chemical compounds. Because of these few cases, it was not worth breaking the entire system of views, which were based on one concept of the atom.
The considered stoichiometric laws form the basis for all kinds of quantitative calculations of the masses and volumes of substances taking part in chemical reactions. In this regard, stoichiometric laws quite rightly relate to the fundamental laws of chemistry. Stoichiometric laws are a reflection of the real existence of atoms and molecules, which, being the smallest particles of chemical elements and their compounds, have a very specific mass. Because of this, stoichiometric laws have become a solid foundation on which modern atomic-molecular science is built.
A) atom B) molecule
A) liquids B) gases
1.solid 2.liquid 3.gas
1. The smallest particle of a substance that retains its properties is
A) atom B) molecule
B) Brownian particle B) oxygen
2. Brownian motion is….
A) chaotic movement of very small solid particles in a liquid
B) chaotic penetration of particles into each other
B) ordered movement of solid particles in a liquid
D) ordered movement of liquid molecules
3. Diffusion can occur...
A) only in gases B) only in liquids and gases
C) only in liquids D) in liquids, gases and solids
4. They do not have their own shape and constant volume...
A) liquids B) gases
C) solids D) liquids and gases
5. Between the molecules there is….
A) only mutual attraction B) only mutual repulsion
C) mutual repulsion and attraction D) there is no interaction
6. Diffusion is faster
A) in solids B) in liquids
C) in gases D) in all bodies the same
7. What phenomenon confirms that molecules interact with each other?
A) Brownian motion B) wetting phenomenon
C) diffusion D) increase in body volume when heated
8. Relate state of aggregation substances and the nature of the movement of molecules:
1.solid 2.liquid 3.gas
A) change their position abruptly
B) fluctuate around a certain point
C) move randomly in all directions
9. Correlate the state of aggregation of a substance and the arrangement of molecules:
1.solid 2.liquid 3.gas
A) randomly, close to each other
B) randomly, the distance is tens of times greater than the molecules themselves
B) molecules are located in in a certain order
10. Correlate the statement about the structure of matter and its experimental substantiation
1. all substances consist of molecules with spaces between them
2. molecules move continuously and randomly
3. molecules interact with each other
A) Brownian motion B) wetting
B) an increase in body volume when heated
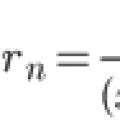 Taylor series expansion Approximate solution of the Cauchy problem for the ordinary
Taylor series expansion Approximate solution of the Cauchy problem for the ordinary What is NOT taught at school What is not taught at school ask
What is NOT taught at school What is not taught at school ask Money Thinking Formula (A
Money Thinking Formula (A