Hydride hydrogen storage system. Transition element hydrides Typical intermetallic hydrides
While the theory of plate tectonics celebrated its “victory”, simultaneously gaining disadvantages in the course of further studies of the structure of the subsoil and moving towards its collapse, the theory of the expansion of the Earth solved its two main problems, and at the same time - a version of such an expansion mechanism was found, which simultaneously removes all questions by “exorbitant” pressures in the core.
A way out of the long impasse was proposed about three decades ago by the Soviet scientist Vladimir Larin (now a Doctor of Geological Sciences), who, as often happens, approached this problem from a completely different angle.
Rice. 69. Diagram of metal and hydrogen atoms
First of all, the dissolution of hydrogen in a metal is not simply mixing it with metal atoms - in this case, hydrogen gives up its electron, which it has only one, to the common treasury of the solution, and remains an absolutely “naked” proton. And the dimensions of a proton are 100 thousand times (!) smaller than the dimensions of any atom, which ultimately (together with the enormous concentration of charge and mass of the proton) allows it to even penetrate deep into the electron shell of other atoms (this ability of a bare proton has already been proven experimentally).
But penetrating inside another atom, a proton seems to increase the charge of the nucleus of this atom, increasing the attraction of electrons to it and thus reducing the size of the atom. Therefore, the dissolution of hydrogen in a metal, no matter how paradoxical it may seem, can lead not to the looseness of such a solution, but, on the contrary, to compaction of the original metal. Under normal conditions (that is, at normal atmospheric pressure and room temperature), this effect is insignificant, but at high pressure and temperature it is quite significant.
Thus, the assumption that the outer liquid core of the Earth contains a significant amount of hydrogen, firstly, does not contradict it chemical properties; secondly, it already solves the problem of deep hydrogen storage for ore deposits; and thirdly, what is more important for us, allows for significant compaction of a substance without an equally significant increase in pressure in it.
“At Moscow University they created a cylinder based on... an intermetallic compound [an alloy of lanthanum and nickel]. Turn the tap and a thousand liters of hydrogen are released from a liter cylinder!” (M. Kuryachaya, “Hydrides that did not exist”).
But it turns out that all these are “seeds”...
In metal hydrides - that is, in chemical compounds of a metal with hydrogen - we have a different picture: it is not hydrogen that gives up its electron (to the general rather loose electronic piggy bank), but the metal gets rid of its outer electron shell, forming a so-called ionic bond with hydrogen. At the same time, the hydrogen atom, accepting an additional electron into the same orbit in which the electron it already has rotates, practically does not change its size. But the radius of a metal atom ion—that is, an atom without its outer electron shell—is significantly smaller than the radius of the atom itself. For iron and nickel, the ion radius is approximately 0.6 of the radius of a neutral atom, and for some other metals the ratio is even more impressive. Such a reduction in the size of metal ions allows them to be compacted in hydride form several times without any increase in pressure as a consequence of such compaction!..
Moreover, this ability to hyper-densify the packing of hydride particles is experimentally detected even under ordinary normal conditions (see Table 1), and at high pressures it increases even more.
Density, g/cm
Metal
Hydride
Compaction, %
Table 1. Compactibility of some hydrides (under normal conditions)
In addition, the hydrides themselves are also capable of dissolving additional hydrogen. At one time they even tried to use this ability in the development of hydrogen car engines for fuel storage.
“...for example, one cubic centimeter of magnesium hydride contains one and a half times more hydrogen by weight than is contained in a cubic centimeter of liquid hydrogen, and seven times more than in a gas compressed to one hundred and fifty atmospheres!” (M. Kuryachaya, “Hydrides that did not exist”).
One problem is that under normal conditions hydrides are very unstable...
But we don’t need normal conditions, since we are talking about the possibility of their existence deep in the bowels of the planet - where the pressure is significantly higher. And with increasing pressure, the stability of hydrides increases significantly.
Nowadays, experimental confirmation of these properties has been obtained, and more and more geologists are gradually inclined to believe that the model of the hydride core may turn out to be much closer to reality than the previous iron-nickel model. Moreover, refined calculations of conditions in the bowels of our planet reveal the unsatisfactory nature of the “pure” iron-nickel model of its core.
“Seismological measurements indicate that both the inner (solid) and outer (liquid) cores of the Earth are characterized by a lower density compared to the value obtained based on a model of a core consisting only of metallic iron under the same physicochemical parameters...
The presence of hydrogen in the core has long been a matter of debate due to its low solubility in iron at atmospheric pressure. However, recent experiments have established that iron hydride FeH can form at high temperatures and pressures and, when plunging deeper, is stable at pressures exceeding 62 GPa, which corresponds to depths of ~1600 km. In this regard, the presence of significant amounts (up to 40 mol.%) of hydrogen in the core is quite acceptable and reduces its density to values consistent with seismological data"(Yu. Pushcharovsky, "Tectonics and geodynamics of the Earth's mantle").
But the most important thing is that under certain conditions - for example, when pressure is reduced or when heated - hydrides are capable of breaking down into their components. Metal ions transform into an atomic state with all the ensuing consequences. A process occurs in which the volume of a substance increases significantly without changing mass, that is, without any violation of the law of conservation of matter. A similar process occurs when hydrogen is released from a solution in a metal (see above).
And this already provides a completely understandable mechanism for increasing the size of the planet!!!
“The main geological and tectonic consequence of the hypothesis of an initially hydrid Earth is a significant, perhaps multiple, over the course of geological history increasing its volume, which is due to the inevitable decompression of the planet’s interior during the degassing of hydrogen and the transition of hydrides to metals” (V. Larin, “Hypothesis of an initially hydride Earth”).
So, Larin proposed a theory that not only solves some of the problems of ore deposits and explains a number of processes in the history of the Earth (to which we will return), but also provides serious ground for the hypothesis of the expansion of our planet - as a side consequence.
Larin did the most important thing - he removed all the main problems of the theory of the expansion of the Earth!..
All that remains are “technical details”.
For example, it is absolutely not clear exactly how much our planet has increased over the entire period of its existence, and at what exact speed its expansion occurred. Different researchers gave estimates that were very different from each other, in addition, strongly reminiscent of a simple sucking of the finger.
“...in the Paleozoic, according to this hypothesis, the radius of the Earth was approximately 1.5 - 1.7 times less than the modern one and, therefore, since then the volume of the Earth has increased approximately 3.5 - 5 times” (O. Sorokhtin, "The Catastrophe of the Expanding Earth").
“The most probable ideas seem to me about a relatively moderate scale of expansion of the Earth, in which from the early Archean (that is, over 3.5 billion years) its radius could have increased by no more than one and a half to two times, from the late Proterozoic (that is, over 1. 6 billion years) - no more than 1.3 - 1.5 times, and from the beginning of the Mesozoic (that is, over the last 0.25 billion years) by no more than 5, maximum 10 percent" (E. Milanovsky, "Earth Is the earth expanding? Is the earth pulsating?").
Alas. Larin's hypothesis also does not directly answer this question.
Moreover, all researchers proceeded from the fact that the process proceeds more or less evenly from the very beginning of the formation of the Earth (the author of the hydride theory, V. Larin, also adheres to this hypothesis). And this leads to such low expansion rates that it is almost impossible to detect it with modern instruments. And testing the validity of the theory seems to be a matter of only the distant future.
It is characteristic that the product of the interaction of hydrogen with thorium, in comparison with the hydrogen derivatives of all other metals, contains greatest number hydrogen and corresponds in composition to the ratio ThH 3.75, i.e., it approaches the composition corresponding to the maximum valence of group IV elements. The density of hydrogen-containing thorium is almost 30% less than the density of the metal, while for other elements of the titanium subgroup the change in density when interacting with hydrogen is approximately 15%.
The simplest hydrides of elements of the carbon subgroup - carbon, silicon, germanium, tin, lead - are tetravalent and correspond to the general formula MeH 4. The thermal stability of hydrides of group IV elements gradually decreases with increasing atomic weight of these elements and atomic radius.
Vanadium subgroup V groups . The interaction of hydrogen with vanadium, niobium and tantalum is largely similar. No chemical compounds of exact stoichiometric composition were found in these systems. Since the absorption and desorption of hydrogen cause irreversible changes in the structure of metallic tantalum, it is possible that in the tantalum-hydrogen system and, apparently, in the niobium-hydrogen system, a certain proportion of intermediate-type chemical bonds are possible.
Simple hydrides of nitrogen, phosphorus, arsenic, antimony and bismuth have general formula MeH3. Hydrides of group V elements are less stable than those of group IV and VI elements. Most elements of group V, in addition to simple hydrides such as NH 3, also form more complex compounds with hydrogen.
From elements of the chromium subgroup Group VI - chromium, molybdenum, tungsten and uranium, only uranium hydride UH 3 has been studied. The chemical bond in this compound is possibly explained by the presence of hydrogen bridges, but not by covalence, which is consistent with the properties of UH 3 . The formation of uranium hydride is accompanied by a sharp (almost 42%) decrease in the density of uranium. This degree of density reduction is the maximum among the studied hydrogen derivatives of metals and, in order of magnitude, corresponds to the increase in density observed during the formation of group I alkali metal hydrides. There is no reliable information about the production of chemical compounds of precise stoichiometric composition by the interaction of hydrogen with chromium, molybdenum and tungsten.
Hydrides of elements of this group can be obtained by direct interaction of elements with hydrogen. In the series H 2 O, H 2 S, H 2 Se, H 2 Te and H 2 Ro, the thermal stability of hydrides quickly decreases.
Regarding the chemical interaction of hydrogen with elements VIII group periodic table - iron, nickel and cobalt - there are conflicting data in the literature. Naturally, doubts arise about the real existence of hydrides of these elements. The interaction of hydrogen with iron, cobalt and nickel at elevated temperatures is not a chemical process in the generally accepted sense. However, this does not yet prove the impossibility of the existence of hydrides of these elements.
Many researchers have reported obtaining products that they believe are hydrides. Thus, there is information about the indirect production of iron hydrides - FeH, FeH 2 and FeH 3, which are stable at temperatures below 150 ° C, above which they decompose. The production of nickel and cobalt hydrides has also been reported. The resulting products were dark, finely dispersed pyrophoric powders. According to some authors, substances of this type are, in fact, not hydrides, but finely dispersed reduced metals containing significant quantities of hydrogen physically adsorbed on the surface. Others believe that adsorbed hydrogen is on the surface of the metal in the atomic state and forms chemical bond with metal atoms.
There is very little consistent data on the chemical interaction of hydrogen with other elements of group VIII (with the exception of palladium).
In table Table 5 shows the available data on the change in the density of metals when interacting with hydrogen.
Let's start with the composition of the injection connections. Let us consider this issue using the example of hydrides of transition elements. If, during the formation of the interstitial phase, hydrogen atoms fall only into tetrahedral voids in the metal lattice, then the limiting hydrogen content in such a compound should correspond to the formula MeH 2 (where Me is a metal whose atoms form a close packing). After all, there are twice as many tetrahedral voids in the lattice as there are atoms forming a close packing. If hydrogen atoms fall only into octahedral voids, then from the same considerations it follows that the limiting hydrogen content should correspond to the formula MeH - there are as many octahedral voids in a dense packing as there are atoms composing this packing.
Typically, when transition metal compounds form with hydrogen, either octahedral or tetrahedral voids are filled. Depending on the nature of the starting materials and the process conditions, complete or only partial filling may occur. In the latter case, the composition of the compound will deviate from the integer formula and will be undefined, for example MeH 1-x; MeN 2-x. Implementation connections, therefore, by their very nature must be compounds of variable composition, i.e., those whose composition, depending on the conditions of their preparation and further processing, varies within fairly wide limits.
Let us consider some typical properties of interstitial phases using the example of compounds with hydrogen. To do this, compare the hydrides of some transition elements with the hydride alkali metal(lithium).
When lithium combines with hydrogen, a substance of a certain composition LiH is formed. By physical properties it has nothing in common with the original metal. Lithium conducts electric current, has a metallic luster, plasticity, in a word, the whole complex metallic properties. Lithium hydride does not have any of these properties. This is a colorless salt-like substance, not at all similar to metal. Like other alkali and alkaline earth metal hydrides, lithium hydride is a typical ionic compound, where the lithium atom has a significant positive charge and the hydrogen atom has an equally negative charge. The density of lithium is 0.53 g/cm 3, and the density of lithium hydride is 0.82 g/cm 3 - occurs noticeable increase in density. (The same is observed during the formation of hydrides of other alkali and alkaline earth metals).
Palladium (a typical transition element) undergoes completely different transformations when interacting with hydrogen. Well known demonstration experience, in which a palladium plate, coated on one side with a gas-proof varnish, bends when blown with hydrogen.
This occurs because the density of the resulting palladium hydride decreases. This phenomenon can only occur if the distance between the metal atoms increases. The introduced hydrogen atoms “push apart” the metal atoms, changing the characteristics of the crystal lattice.
The increase in the volume of metals upon absorption of hydrogen with the formation of interstitial phases occurs so noticeably that the density of the metal saturated with hydrogen turns out to be significantly lower than the density of the original metal (see Table 2)
Strictly speaking, the lattice formed by metal atoms usually does not remain completely unchanged after the absorption of hydrogen by this metal. No matter how small the hydrogen atom is, it still introduces distortions into the lattice. In this case, there is usually not just a proportional increase in the distances between atoms in the lattice, but also some change in its symmetry. Therefore, it is often said only for simplicity that hydrogen atoms are introduced into voids in a dense packing - the dense packing of metal atoms itself is still disrupted when hydrogen atoms are introduced.
Table 2 Change in the density of some transition metals during the formation of interstitial phases with hydrogen.
This is far from the only difference between hydrides of typical and transition metals.
During the formation of interstitial hydrides, such typical properties of metals as metallic luster and electrical conductivity are preserved. True, they may be less pronounced than in the parent metals. Thus, interstitial hydrides are much more similar to the parent metals than alkali and alkaline earth metal hydrides.
Such a property as plasticity changes significantly more - metals saturated with hydrogen become brittle, often the original metals are difficult to turn into powder, but with hydrides of the same metals this is much easier.
Finally, we should note a very important property of interstitial hydrides. When transition metals interact with hydrogen, the metal sample is not destroyed. Moreover, it retains its original shape. The same happens during the reverse process - the decomposition of hydrides (loss of hydrogen).
A natural question may arise: can the process of formation of interstitial phases be considered chemical in the full sense of the word? Is it possible that aqueous solutions are formed - a process that has much more “chemistry”?
To answer, we need to use chemical thermodynamics.
It is known that the formation of chemical compounds from simple substances (as well as other chemical processes) is usually accompanied by noticeable energy effects. Most often, these effects are exothermic, and the more energy released, the stronger the resulting connection.
Thermal effects are one of the most important signs that not just a mixing of substances is occurring, but a chemical reaction is taking place. Once the internal energy of the system changes, therefore, new connections are formed.
Let us now see what energetic effects are caused by the formation of interstitial hydrides. It turns out that the spread here is quite large. In metals of side subgroups III, IV and V of groups of the periodic system, the formation of interstitial hydrides is accompanied by a significant release of heat, on the order of 30-50 kcal/mol (when lithium hydride is formed from simple substances, about 21 kcal/mol is released). It can be admitted that interstitial hydrides, at least of the elements of the indicated subgroups, are quite “real” chemical compounds. It should be noted, however, that for many metals located in the second half of each transition series (for example, iron, nickel, copper), the energetic effects of the formation of interstitial hydrides are small. For example, for a hydride of the approximate composition FeH 2, the thermal effect is only 0.2 kcal/mol .
The small value of the DN of such hydrides dictates the methods for their preparation - not the direct interaction of the metal with hydrogen, but an indirect way.
Let's look at a few examples.
Nickel hydride, the composition of which is close to NiH 2, can be obtained by treating an ethereal solution of nickel chloride with phenylmagnesium bromide in a stream of H 2:
The nickel hydride obtained as a result of this reaction is a black powder that easily gives off hydrogen (which is generally characteristic of interstitial hydrides); when slightly heated in an oxygen atmosphere, it ignites.
In the same way, hydrides of nickel's neighbors can be obtained. periodic table- cobalt and iron.
Another method for preparing transition hydrides is based on the use of lithium alanate LiAlH. When the chloride of the corresponding metal reacts with LiAlH 4 in an ethereal solution, an alanate of this metal is formed:
MeCl 2 +LiAlH 4 >Me(AlH 4 ) 2 +LiCl(5)
For many metals, alanates are fragile compounds that decompose when the temperature increases.
Me(AlH 4 ) 2 >MeH 2 + Al + H 2 (6)
But for some metals of secondary subgroups, a different process occurs:
Me(AlH 4 ) 2 >MeH 2 +AlH 3 (7)
In this case, instead of a mixture of hydrogen and aluminum, aluminum hydride is formed, which is soluble in ether. By washing the reaction product with ether, a pure transition metal hydride can be obtained as a residue. In this way, for example, low-stable hydrides of zinc, cadmium and mercury were obtained.
It can be concluded that the preparation of hydrides of elements of side subgroups is based on typical methods of inorganic synthesis: exchange reactions, thermal decomposition of fragile compounds under certain conditions, etc. By these methods, hydrides of almost all transition elements, even very fragile ones, were obtained. The composition of the resulting hydrides is usually close to stoichiometric: FeH 2, CoH 2, NiH 2 ZnH 2, CdH 2, HgH 2. Apparently, the achievement of stoichiometry is facilitated by the low temperature at which these reactions are carried out.
Let us now examine the influence of reaction conditions on the composition of the resulting interstitial hydrides. It follows directly from Le Chatelier's principle. The higher the hydrogen pressure and the lower the temperature, the closer the saturation of the metal with hydrogen is to the limiting value. In other words, each certain temperature and each pressure value corresponds to a certain degree of saturation of the metal with hydrogen. Conversely, each temperature corresponds to a certain equilibrium pressure of hydrogen above the metal surface.
This is where one of the possible applications of transition element hydrides comes from. Let's say that in some system you need to create a strictly defined hydrogen pressure. A metal saturated with hydrogen is placed in such a system (titanium was used in the experiments). By heating it to a certain temperature, you can create the required pressure of hydrogen gas in the system.
Any class of compounds is interesting in its own way chemical nature, the composition and structure of the particles of which it consists and the nature of the connection between these particles. Chemists devote their theoretical and experimental work to this. They are no exception from the implementation phase.
There is no definitive point of view on the nature of interstitial hydrides yet. Often different, sometimes opposing points of view successfully explain the same facts. In other words, there are no unified theoretical views on the structure and properties of interstitial compounds yet.
Let's consider some experimental facts.
The process of hydrogen absorption by palladium has been studied in most detail. It is characteristic of this transition metal that the concentration of hydrogen dissolved in it at a constant temperature is proportional to the square root of the external hydrogen pressure.
At any temperature, hydrogen, to some extent, dissociates into free atoms, so there is an equilibrium:
The constant for this equilibrium is:
Where R N -- pressure (concentration) of atomic hydrogen.
From here (11)
It can be seen that the concentration of atomic hydrogen in the gas phase is proportional to the square root of the pressure (concentration) of molecular hydrogen. But the concentration of hydrogen in palladium is also proportional to the same value.
From this we can conclude that palladium dissolves hydrogen in the form of individual atoms.
What, then, is the nature of the bond in palladium hydride? To answer this question, a number of experiments were carried out.
It was found that when passing electric current through hydrogen-saturated palladium, non-metal atoms move to the cathode. It must be assumed that the hydrogen found in the metal lattice is completely or partially dissociated into protons (i.e., H + ions) and electrons.
Information about electronic structure palladium hydride were obtained by studying the magnetic properties. The change in the magnetic properties of the hydride depending on the amount of hydrogen entering the structure was studied. Based on the study of the magnetic properties of a substance, it is possible to estimate the number of unpaired electrons contained in the particles of which this substance consists. On average, there are approximately 0.55 unpaired electrons per palladium atom. When palladium is saturated with hydrogen, the number of unpaired electrons decreases. And in a substance with the composition PdH 0.55, there are practically no unpaired electrons.
Based on these data, we can conclude: unpaired electrons of palladium form pairs with unpaired electrons of hydrogen atoms.
However, the properties of interstitial hydrides (in particular, electrical and magnetic) can also be explained on the basis of the opposite hypothesis. It can be assumed that interstitial hydrides contain H - ions, which are formed due to the capture by hydrogen atoms of part of the half-free electrons present in the metal lattice. In this case, the electrons obtained from the metal would also form pairs with the electrons present on the hydrogen atoms. This approach also explains the results of magnetic measurements.
It is possible that both types of ions coexist in interstitial hydrides. Metal electrons and hydrogen electrons form pairs and, therefore, a covalent bond. These electron pairs can be shifted to one degree or another towards one of the atoms - metal or hydrogen.
The electron pair is biased more toward the metal atom in hydrides of those metals that are less likely to donate electrons, such as palladium or nickel hydrides. But in scandium and uranium hydrides, apparently, the electron pair is strongly shifted towards hydrogen. Therefore, hydrides of lanthanides and actinides are in many ways similar to hydrides of alkaline earth metals. By the way, lanthanum hydride reaches the composition LaH 3. For typical interstitial hydrides, the hydrogen content, as we now know, is not higher than that corresponding to the formulas MeH or MeH 2.
Another experimental fact shows the difficulties of determining the nature of the bond in interstitial hydrides.
If hydrogen is removed from palladium hydride at a low temperature, it is possible to retain the distorted (“expanded”) lattice that palladium saturated with hydrogen had. The magnetic properties (note this), electrical conductivity and hardness of such palladium are generally the same as those of the hydride.
It follows that during the formation of interstitial hydrides, the change in properties is caused not only by the presence of hydrogen in them, but also simply by a change in interatomic distances in the lattice.
We have to admit that the question of the nature of interstitial hydrides is very complex and far from being finally resolved.
Humanity has always been famous for the fact that, even without fully knowing all aspects of any phenomena, it was able to practically use these phenomena. This fully applies to interstitial hydrides.
The formation of interstitial hydrides in some cases is deliberately used in practice, in other cases, on the contrary, they try to avoid it.
Interstitial hydrides give off hydrogen relatively easily when heated and sometimes at low temperatures. Where can I use this property? Of course, in redox processes. Moreover, the hydrogen released by interstitial hydrides is in an atomic state at some stage of the process. This is probably related to the chemical activity of interstitial hydrides.
It is known that group eight metals (iron, nickel, platinum) are good catalysts for reactions in which hydrogen attaches to any substance. Perhaps their catalytic role is associated with the intermediate formation of unstable interstitial hydrides. By further dissociating, the hydrides provide the reaction system with a certain amount of atomic hydrogen.
For example, finely dispersed platinum (the so-called platinum black) catalyzes the oxidation of hydrogen with oxygen - in its presence, this reaction proceeds at a noticeable speed even at room temperature. This property of platinum black is used in fuel cells - devices where chemical reactions are used to directly produce electrical energy, bypassing the production of thermal energy (combustion stage). The so-called hydrogen electrode, an important tool for studying the electrochemical properties of solutions, is based on this same property of finely dispersed platinum.
The formation of interstitial hydrides is used to obtain highly pure metal powders. Uranium metal and other actinides, as well as very pure titanium and vanadium, are ductile, and therefore it is practically impossible to prepare powders from them by grinding the metal. To deprive the metal of its ductility, it is saturated with hydrogen (this operation is called “embrittlement” of the metal). The resulting hydride is easily ground into powder. Some metals, even when saturated with hydrogen, themselves turn into a powder state (uranium). Then, when heated in a vacuum, the hydrogen is removed and what remains is pure metal powder.
The thermal decomposition of some hydrides (UH 3, TiH 2) can be used to produce pure hydrogen.
The most interesting areas of application of titanium hydride. It is used for the production of foam metals (for example, aluminum foam). To do this, the hydride is introduced into molten aluminum. At high temperatures, it decomposes, and the resulting hydrogen bubbles foam the liquid aluminum.
Titanium hydride can be used as a reducing agent for some metal oxides. It can serve as solder for joining metal parts, and as a substance that accelerates the sintering process of metal particles in powder metallurgy. In the last two cases they are also used restorative properties hydride. A layer of oxides usually forms on the surface of metal particles and metal parts. It prevents adhesion of adjacent sections of metal. When heated, titanium hydride reduces these oxides, thereby cleaning the metal surface.
Titanium hydride is used to produce some special alloys. If it is decomposed on the surface of a copper product, a thin layer of copper-titanium alloy is formed. This layer gives the surface of the product special mechanical properties. Thus, you can combine several in one product important properties(electrical conductivity, strength, hardness, abrasion resistance, etc.).
Finally, titanium hydride is a very effective means of protecting against neutrons, gamma rays and other hard radiation.
Sometimes, on the contrary, one has to fight against the formation of interstitial hydrides. In metallurgy, chemical, oil and other industries, hydrogen or its compounds are under pressure and at high temperatures. Under such conditions, hydrogen can diffuse to a noticeable extent through the heated metal and simply “leave” from the equipment. In addition (and this is perhaps most important!), due to the formation of interstitial hydrides, the strength of metal equipment can be greatly reduced. And this already poses a serious danger when working with high pressures.
By storing hydrogen in hydride form, there is no need for bulky and heavy cylinders required when storing compressed hydrogen gas, or difficult to manufacture and expensive vessels for storing liquid hydrogen. When storing hydrogen in the form of hydrides, the volume of the system is reduced by approximately 3 times compared to the volume of storage in cylinders. Hydrogen transportation is simplified. There are no costs for conversion and liquefaction of hydrogen.
Hydrogen can be obtained from metal hydrides by two reactions: hydrolysis and dissociation:
By hydrolysis it is possible to obtain twice as much hydrogen as is present in the hydride. However, this process is practically irreversible. The method of producing hydrogen by thermal dissociation of a hydride makes it possible to create hydrogen accumulators, for which a slight change in temperature and pressure in the system causes a significant change in the equilibrium of the hydride formation reaction.
Stationary devices for storing hydrogen in the form of hydrides do not have strict restrictions on mass and volume, so the limiting factor in choosing a particular hydride will, in all likelihood, be its cost. For some applications, vanadium hydride may be useful, since it dissociates well at a temperature close to 270 K. Magnesium hydride is relatively inexpensive, but has a relatively high dissociation temperature of 560-570 K and a high heat of formation. The iron-titanium alloy is relatively inexpensive, and its hydride dissociates at temperatures of 320-370 K with a low heat of formation.
The use of hydrides has significant safety advantages. A damaged hydrogen hydride vessel poses significantly less danger than a damaged liquid hydrogen tank or pressure vessel filled with hydrogen.
It is important that the binding of hydrogen to a metal occurs with the release of heat. The exothermic process of formation of a hydride from hydrogen M of a metal (charging) and the endothermic process of releasing hydrogen from the hydride (discharging) can be represented in the form of the following reactions:

For the technical use of hydrides, temperatures at which the pressure of hydrogen dissociation in the hydride reaches values above 0.1 MPa are of particular interest. Hydrides in which the dissociation pressure above 0.1 MPa is achieved at a temperature below the freezing point of water are called low-temperature. If this pressure is achieved at a temperature above the boiling point of water, then such hydrides are considered high-temperature.
For the needs of road transport, hydrides are created, which theoretically can contain up to 130-140 kg of hydrogen per 1 m 3 of metal hydride. However, the realized hydride capacity is unlikely to exceed 80 kg/m 3 But even this hydrogen content in a tank with a capacity of 130 dm 3 is sufficient for 400 km of vehicle mileage. These are realistic indicators for use, but the increase in mass of the tank filled with hydride should be taken into account. For example, the mass of lathan-nickel hydride reaches 1 ton, and magnesium hydride - 400 kg.
To date, metal hydrides with a wide range of properties have been synthesized and studied. Data on the properties of some hydrides that are of greatest potential interest for industrial use are given in Table. 10.3 and 10.4. As can be seen from table. 10.3, for example, magnesium hydride makes it possible to store 77 g of H2 per 1 kg of hydride mass, while in a cylinder under a pressure of 20 MPa there is only 14 g per 1 kg of container. In the case of liquid hydrogen, you can store 500 g per 1 kg container.
The Comprehensive Program of Search, Research and Development Work on Hydrogen Energy and Fuel Cells plans to study palladium. The platinum group metal palladium is one of the main materials for fuel cells and all hydrogen energy. On its basis, catalysts, membrane devices for producing pure hydrogen, and materials with increased functional characteristics, fuel cells, electrolyzers, sensors for hydrogen determination. Palladium can effectively accumulate hydrogen, especially palladium nanopowder.
In addition to hydrogen energy, palladium is used in catalysts for post-treatment of exhaust gases from conventional cars; electrolyzers for producing hydrogen and oxygen by decomposing water; portable fuel cells, in particular methanol; solid oxide electrolyzers with palladium-based electrodes; devices for obtaining oxygen from air, including for medical purposes; sensors for the analysis of complex gas mixtures.
It is important to note that our country controls about 50% of the world production of this metal necessary for the production of hydrogen. Currently, at the Institute of Chemical Physics of the Russian Academy of Sciences in Chernogolovka, work is underway to create hydrogen batteries based on metal hydrides.
Properties of some hydrides
Table 10.3
Let us name several distinctive characteristics of materials used in hydride systems.
1) All alloys bearing the HY-STOR trademark are manufactured by Energies, Inc. Much of the data given in this paragraph is taken from the work of Huston and Sandrock. In chemical formulas, the symbol M stands for mischmetal, a mixture of rare earth metals usually obtained from monazite dust. The effect of mischmetal on plateau pressure strongly depends on the ratio of the amounts of cerium and lanthanum in this mixture of metals.
Plateau slope
In accordance with the simplified thermodynamic model of the hydride system described in the next paragraph, the plateau in the equilibrium dependence | pressure from concentration should be horizontal. However, in practice; the pressure on the plateau increases slightly with increasing hydrogen concentration in the solid phase.
The slope of the plateau can be quantitatively characterized by the slope coefficient d n(pd)/d(H, M), where pd is the pressure at the plateau on the desorption isotherm. In Fig. 9.7, the dotted line passing through the desorption isotherm corresponding to 25 °C intersects the vertical line H/M = 0 at the point pd = 9.1 atm, and the line H/M = 1.2 at the point pd = 14.8 atm. Then
dlnpd In 14.8-In 9.1
M) 1.2 ' ■ U '
This coefficient value is acceptable. The slope parameter of the equilibrium pressure plateau for the TiFe alloy, for example, is zero, while for some calcium alloys the value of this parameter exceeds three. When the alloy solidifies (at the manufacturing stage), there is a tendency to segregation, i.e., the release of some elements that make up the alloy. Apparently, this phenomenon is the main reason for the appearance of the plateau slope, since from the standpoint of thermodynamics, the dependence of the equilibrium pressure on the hydrogen concentration for an ideally homogeneous alloy should have a horizontal plateau. Annealing the material before grinding it can reduce the slope of the plateau. The values of the slope coefficient and some other characteristics are given in table. 9.4, 9.5 and 9.6.
Absorption-desorption hysteresis
As noted above, the plateau pressure during absorption is usually slightly higher than during desorption. In other words, hysteresis of absorption and desorption processes is observed during cyclic charging and discharging of the alloy (see Fig. 9.7,
9.8, 9.10 and 9.11).
|
Table 9.4. Thermodynamic properties of some metal hydrides
|
|
Plateau slope8*, ^ |
Hysteresis coefficient Pa/Pd |
||
|
Table 9.6. Maximum hydrogen content and heat capacity of some metal hydrides
|
The phenomenon of hysteresis is associated with the irreversible process of heat release due to plastic deformation of the crystal lattice, namely its expansion during absorption and compression during desorption of hydrogen.
The phenomenon of hysteresis is quantitatively characterized by the ratio of the values of the equilibrium pressure of hydrogen during absorption and desorption at a value of AHM = 0.5 and usually a temperature of 25 °C. It is generally accepted that this ratio does not depend on temperature.
The useful capacity is defined as the change in the number of absorbed hydrogen atoms per metal atom in the hydride, N/M, when the pressure changes from a value of 10 times the plateau pressure to a value of 0.1 plateau pressure. This method of determining useful capacity gives slightly overestimated values. A more realistic value is obtained if the pressure range is significantly narrowed.
In Fig. 9.9 (Fe0 8ІЧІ(| 2Ті) alloy) the pressure on the plateau at a temperature of 70 °C is approximately 0.9 atm. At a pressure 10 times greater than the indicated value, the N/M ratio is 0.65, and at a pressure 10 times less than pressure on the plateau, N/M = 0.02. Thus, the difference A(N/M) = 0.63. In other words, 0.63 kmol of atomic hydrogen (0.63 kg) can be extracted from 1 kmol of hydride.
FeTi alloy (cf. Fig. 9.4)
Heat capacity
Hydride systems are activated by temperature changes. In order to design such systems, it is necessary to have information about the heat capacity of various alloys. The heat capacity values for a number of alloys are given in Table. 9.6.
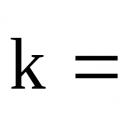 Reaction rate constant Guidelines for laboratory work
Reaction rate constant Guidelines for laboratory work Is there life after death?
Is there life after death? Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe
Scientists: Earth is a “space prison” for humanity scientists: Earth is a space prison in the universe